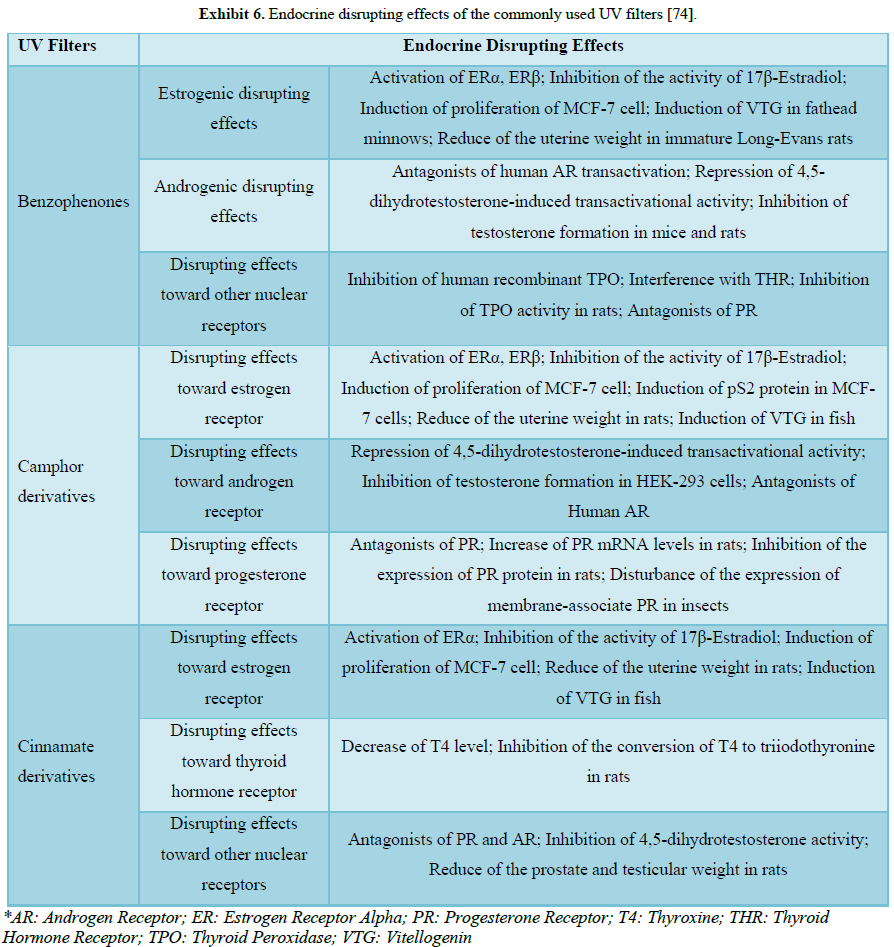
9073
Views & Citations8073
Likes & Shares
The
sunscreen industry is achieving remarkable worldwide prominence by responding
to the growing need for skin protection with fast-paced innovation. Increased
consumer awareness of the harmful effects of sunlight has fueled the demand for
improved photo protection. The need for broad-spectrum protection from both UVA
and UVB rays has inspired scientists worldwide to research new cosmetic
formulations and delivery systems. More effective sunscreen actives, emollients
and novel cosmetic and functional ingredients have been regularly added to the
formulator’s repertoire. Creativity in innovation has been hindered only by
regulatory agencies and patent restrictions worldwide. Familiarity with the
current restrictive regulations and patent law infringements has become
integral to any research effort attempting to provide improved protection to
individuals affected by the sun’s damaging effects. The increasing incidence of
skin cancers and photo damaging effects caused by ultraviolet radiation has
increased the use of sun screening agents, which have shown beneficial effects
in reducing the symptoms and reoccurrence of these problems. Unlike the
situation in Europe where sunscreen ingredients are considered under cosmetics
guidelines, the FDA is required to define sunscreens as drugs since they are
advertised to prevent sunburn and, more recently, the risk of skin cancer. In
the USA, the FDA has been regulating this industry since August 25, 1978, with
the publication of the Advance Notice of Proposed Rulemaking. Sunscreens are
considered drugs and cosmetics and therefore must be governed by the FDA-OTC
monograph. With the variety of sunscreen agents used in cosmetic and UV
protection products, Australia, Canada, and the European Union (EU) have also
developed regulatory protocols on safe sunscreen product use. Unlike the USA
though, Australia has approved 34 active sunscreen ingredients and the EU has
approved 28 of these ingredients. Current FDA regulations allow labeling of
sunscreen products to a maximum of 30þ, despite the many products currently
available with numbers as high as 100. From a cosmetic formulation point of
view, increasing the SPF number in a product is governed by simple chemical
principles.
Keywords: Melanoma, Carcinoma,
Immunosuppression, Retinoids, Tretinoin, Photodamage
Abbreviations: NMSC: Non-Melanoma Skin Cancer; KLF4: Kruppel
Like Factor 4; PPARG: Peroxisome Proliferator Activated Receptor Gamma; APC: Antigen
Presenting Cell; BBB: Blood-Brain Barrier; EWG: Environmental Working Group; GRASE:
Generally Recognized as Safe and Effective; PABA: P-Aminobenzoic Acid; MSs: Mesoporous
Silicas; HPCD: Hydroxypropyl-Beta-Cyclodextrin; OMC: Octyl Methoxycinnamate; ROS:
Reactive Oxygen Species; PUVA: Psoralen Plus UVA; SAD: Seasonal Affective
Disorder; MDD: Major Depressive Disorder; AK: Actinic Keratosis; SCC: Squamous
Cell Carcinoma; BCC: Basal Cell Carcinoma; NPs: Polymeric Nanoparticles; BEMT: Bisethylhexyloxyphenol
Methoxyphenyl Triazine; SLN: Solid Lipid Nanoparticle; HDPE/LDPE: High-Density
Polyethylene/Low-Density Polyethylene; EAE: Autoimmune Encephalomyelitis; MS: Multiple
Sclerosis; iNOS: Inducible Nitric Oxide Synthase; TNF- α: Tumor Necrosis Factor
Alpha; IARC: International Agency for Research on Cancer; UVAPF: Ultraviolet
(UV) A Protection Factor
BACKGROUND
Ra (Re) was the primary name of the sun god of Ancient Egypt. According to Osiris myth, Nut, the mother of Osiris swallowed the setting sun (Ra) each evening and gave birth to him each morning. The Ancient Egyptians were well aware of the dangers of the sun. Their lands were scorched with heat. Women protected their skin, preferring light skin to dark in their cultural hierarchy of beauty. Recent discoveries written on papyri and the walls of several tombs unearthed ingredients and formulations in use in Ancient Egypt specifically addressing issues of sun damage to the hair and skin. Also, a brief historical review manifests the following interesting things indeed (Figure 1):
·
Jasmin was used to heal the sun-damaged
skin. Recent evidence reveals that Jasmin aids in DNA repair at the cellular
level.
·
Aloe was used to heal sun-damaged skin.
·
Olive oil was used as hydrating oil for
both skin and hair damaged by overexposure to the sunlight.
·
Almond oil was applied before and after
sun exposure to hydrate the sun-damaged skin, improving elasticity and texture.
·
Rice bran extracts were used in
sunscreen preparations. Today, gamma oryzanol extracted from rice bran has UV
absorbing properties.
·
Kohl (to darken eyes in order to combat sunlight impairment to the retina
in the glare of the desert sun), red ochre (to redden and impart a rosy glow in
women’s makeup mimicking the effect of the sun on the skin), and henna oil (to
dye the lips and nails, darken the color of the hair and skin and protect light
skin from the sun). Today, henna is one of the most widely used natural
sunscreen with both UVA and UVB protection.
·
Lupin extract was used to block the rays of the sun and is still used to
date to lighten the color of the skin.
·
Calcite powder and clay were used as UV filters similar to the modern-day
inorganic particulates zinc oxide and titanium dioxide.
·
Aquatic lotus oil was used for protection of the skin from the sun.
INTRODUCTION
Protection from sunlight is often equated with use of sunscreens, but
this approach is too narrow, and protection should consist of a package of
measures: avoiding overexposure to sunlight, using sunscreens, and wearing
protective clothing. Solar ultraviolet (UV) radiation significantly influences
the skin, causing aging, sunburns, precancerous and cancerous lesions and
immunosuppression. UV radiation has an immunosuppressive effect on the
antigen-presenting cells within the epidermis and contributes to the likelihood
of skin cancer. If solar radiation is a primary risk factor for malignant
melanoma, it is reasonable to conclude that reducing sun exposure via topical
sunscreen use would be associated with reduced disease risk. Melanoma is more
common in Whites than in Blacks and Asians. The incidence of non-melanoma skin
cancer (NMSC) is dramatically increasing worldwide, despite the increased use
of improved sunscreens. In 2014, the Surgeon General estimated that 2.2-5.0
million people were treated annually for NMSC. As the number of newly diagnosed
skin cancers continues to rise, there is a need for additional preventative
measures beyond sunscreens. UV radiation is the second most prevalent
carcinogenic exposure in Canada, fifth in Switzerland and is similarly
important in other countries with large Caucasian populations. Within the UK,
Wales has among the highest rates of skin cancer annually and skin cancer
diagnosis rates have increased 63% in 10 years. Australia and New Zealand
having the world's highest skin cancers. People living in Australia and New
Zealand are now advised to apply sunscreen every day when the UV index is
predicted to reach 3 or above. Denmark has one of the highest incidences of
melanoma in the world, although it is a relatively northern country.
Implications for public health: Increased use of sunscreen as part of the daily
routine to reduce incidental sun exposure will lead to decreased incidence of
skin cancer in the future. There are 3 kinds of UV radiation: UVC, UVB, and
UVA. The ozone layer ingests 100% of UVC, 90% of UVB and a negligible measure
of UVA. Therefore, exhaustion of the ozone layer expands UV transmission. UVA
is related with aging and pigmentation. It enters profound into the skin layer
and creates free extreme oxygen species, in a roundabout way damaging DNA. UVA
expands the quantity of incendiary cells in the dermis and diminishes the
quantity of antigens introducing cells. UVB causes sunburn and DNA strand
breaks. It causes pyrimidine dimer changes which are related with non-melanoma
skin malignancies. Photo protection includes both essential and secondary
protective variables. Essential variables are sunscreens; these incorporate
physical barriers which reflect and dissipate light and substance barriers
which ingest light. Secondary components incorporate cancer prevention agents,
osmolytes and DNA repair enzymes which help to constrain skin harm by
exasperating the photochemical cascade that happens by UV sunlight. People with
black skin are much less susceptible to sunburn than white-skinned individuals.
Black people living in the UK were more likely to use sunscreen as a form of
sun protection, whereas sunscreen was the least popular modality in the two
African countries with shade being the most common form of limiting sun
exposure. A significant benefit from regular sunscreen use has not yet been
demonstrated for primary prevention of basal cell carcinoma and melanoma.
Concerning the prevention of actinic keratoses, squamous cell carcinomas, and
skin aging, the effect of sunscreens is significant, but it remains incomplete.
Some organic UV filters (PABA derivatives, cinnamates, benzophenones and
octocrylene) have been described to cause photo allergy. Percutaneous
absorption and endocrine disrupting activity of small-sized organic and
nano-sized inorganic UV filters have been reported.
POSITIVE EFFECTS OF
UVR
Exposure to UVR is not always considered bad. In fact, UVR has been found
to be particularly helpful in treating vitamin D deficiency, seasonal affective
disorders, psoriasis, sarcoidosis, mycosis fungoides and numerous other
cutaneous conditions. Within the epidermis, 7-dehydrocholesterol is converted
to vitamin D (cholecalciferol) by UVB light. The elderly and young children are
the ones who are particularly susceptible to vitamin D deficiency. Vitamin D
deficiency can lead to rickets in children, osteomalacia in adults,
osteopenia/osteoporosis, and factures in the elderly. The Institute of Medicine
recommends the following vitamin D allowances: 400 IU for 0 to 12 months, 600
IU for 1 to 70 years and 800 IU for greater than 70 years. Light therapy is an
inexpensive treatment and can be beneficial in treating certain diseases.
Although it has been known for the last 100 years that UV light (particularly
UVC with a wavelength range of 240-280 nm) is highly germicidal, its use to
treat wound or other localized infections remains at an early stage of
development. Candida auris is
globally emerging yeast, causing severe infections in patients with underlying
diseases. This yeast is responsible for several outbreaks within healthcare
facilities, where it can be found on hospital surfaces and patient care
devices. Spread from these fomites may be prevented by improving the
decontamination of hospital surfaces. UV-C decontamination may constitute an
effective adjunct to routine room cleaning. Additionally, difficult-to-treat
psoriasis patients sometimes find relief with UVR. It is thought that UVR has
both anti-proliferative and anti-inflammatory effects through down regulation
of T-cell response to antigens. Studies have also shown improvement of the
cutaneous effects of sarcoidosis with UVA-1 light and topical psoralen plus UVA
(PUVA) therapy. PUVA and narrowband UVB has been shown to induce and maintain
remissions of mycosis fungoides. Phototherapy is the use of light for reducing
the concentration of bilirubin in the body of infants. However, the exposure to
UV in childhood has been established as an important contributing factor for
melanoma risk in adults and considering the high susceptibility to UV-induced
skin damage of the new-born, related to his pigmentary traits, the UV exposure
of the infant during phototherapy should be “as low as reasonably achievable,”
considering that it is unnecessary to the therapy. Protective eyewear can be
necessary during new-born assistance activities carried out in proximity of
some sources. Most people judge sun exposure in non-erythemic doses as
pleasant. Exposure to sunlight has been linked to improved energy and elevated
mood. Seasonal affective disorder (SAD) is a seasonal pattern of recurrent
major depressive episodes that most commonly occurs during autumn or winter and
remits in spring. The prevalence of SAD ranges from 1.5% to 9%, depending on
latitude. Evidence on light therapy as preventive treatment for patients with a
history of SAD is limited, preventive treatment of SAD and the treatment
selected should be strongly based on patient preferences. Light therapy, an
effective treatment for seasonal affective disorder (SAD), may also be
appropriate for MDD which is the second-ranked cause of disability worldwide.
Bright light treatment, both as monotherapy and in combination with fluoxetine,
was efficacious and well tolerated in the treatment of adults with non-seasonal
MDD. The combination treatment had the most consistent effects [77-83].
SUNBURN
Sunburn and sunscreen
facts
·
Sunburn and sun poisoning are forms of skin damage due to UV ray
exposure. The symptoms can range from mild to severe and may require treatment.
·
Sunburn might be sun poisoning if there is blisters, hives or rash, fever
and chills, nausea, dehydration, headache, pain and tingling, vision problems
[1].
·
UV rays are most intense at noon and the hours immediately before and
after (between 10 AM and 4 PM) [2-10].
·
Immediate symptoms of sunburn are hot, red, tender skin; pain when the
skin is touched or rubbed; and dehydration; several days after exposure the
skin may swell, blister and peel [3].
·
Most sunburns are mild and can be treated with home remedies such as
applying damp cloths or compresses to reduce the pain, soaking in a tepid bath
(with no soap), gently patting the skin dry, applying soothing creams or
lotions, OTC pain relievers such as Tylenol or others, and moisturizing the
skin [4].
·
Sunburn may cause permanent skin damage and skin cancer (malignant
melanoma, basal cell carcinoma, squamous cell carcinoma) [5].
·
Persons with certain pigment disorders and individuals with fair skin are
at most risk of sunburn [6].
·
Certain diseases and conditions pose a higher risk of sunburn (for
example, albinism, lupus, porphyria, vitiligo and xeroderma pigmentosum) [7].
·
Some medications may increase sensitivity to sunburn (photosensitivity)
[8,10].
·
Sun poisoning is caused by severe sunburn; its symptoms include fever,
nausea, chills, dizziness, rapid pulse, rapid breathing, dehydration and shock
[9].
·
UVA and UVB are mainly responsible for skin pathologies such as sunburns,
cutaneous degeneration, photosensitivity, phototoxicity, photo-aging,
immunosuppression and skin cancer [11-27].
·
Snow reflects up to 80% of the sun’s rays, sand reflects 15%, and grass,
soil and water reflect 10%. Due to these factors, an individual sitting under a
solitary standard beach umbrella can be exposed to up to 84% of the total UV
radiation despite feeling adequately covered. For these reasons, it is best to
seek deep shade [28].
·
Less than 50% of the SPF number claimed on the label is spread on the
consumer's skin, meaning that a sunscreen with an SPF 30 will give the real
protection of an SPF of 15. Therefore, SPF 60 should be recommended if real
protection of 30 is desired. Significant injury, DNA damage, mutations and
carcinogenesis can and do occur also with cumulative sub-erythemal UV exposure
[29].
·
The SPF was created in 1956 by Schulze and it reflects the ratio between
the lower amounts of UV energy required producing a minimal erythema on
sunscreen protected skin and the amount of energy required to produce the same
erythema on unprotected skin [30,31].
·
Since many of UV filters were shown to cross the blood-brain barrier (BBB),
the risk for neurotoxicity also occurs [32-37].
·
Sunscreen compounds might block vitamin D synthesis or act as endocrine
disruptor and lead to developmental toxicity. The effects of sunscreen on
cutaneous synthesis of vitamin D induced by sunlight have been a subject of
debate for recent years; however the newest analysis suggests that normal usage
of sunscreen by adults do not decrease cutaneous synthesis of vitamin D [38].
·
The FDA is notoriously slow in approving sunscreen compounds, requiring
exhaustive evidence of their safety. Indeed, FDA has approved only 16 sunscreen
ingredients (14 organic filters and two nonorganic filters, including zinc
oxide and titanium dioxide), while other areas of the world, like the European
Union and Australia, have approved nearly twice as many [39].
·
FDA approved sunscreen ingredients are Aminobenzoic acid, Avobenzone,
Cinoxate, Dioxybenzone, Homosalate, Meradimate, Octocrylene, Octinoxate,
Octisalate, Oxybenzone, Padimate O, Ensulizole, Sulisobenzone, Titanium
dioxide, Trolamine salicylate and Zinc oxide [40-44].
·
2 of the 16 main ingredients used in OTC sunblock products are safe, the
FDA said. Moreover, the FDA is requesting more information on 12 ingredients
among the 16 [45].
·
The FDA has changed its guidelines to address broad-spectrum sunscreen
use, which involves UV-A and UV-B coverage; water resistance, to indicate the
time duration the sunscreen is effective; and sun protection factor (SPF).
SPF-15 or higher is recommended and can be labeled as reducing the risk of skin
cancer and early skin aging [40].
·
PABA and trolamine salicylate — are not GRASE for use in sunscreens, are
no longer permitted for use in OTC sunscreen products. No sunscreens sold in
the United States contain PABA or trolamine salicylate [41,42].
·
For a decade, Environmental Working Group (EWG) has worked to raise
concerns about sunscreens with oxybenzone, which is found in nearly all
Americans, detected in breast milk and potentially causing endocrine disruption
[43].
·
Hawaii recently enacted legislation that will ban the use of two major
ingredients -oxybenzone and octinoxate-that have also been implicated in coral
toxicity and will be banned. This creates a healthcare dilemma: Will the
protection of coral reefs result in an increase in human skin cancers? [46-51].
Sunburn:
Pathophysiology
Sunburn is a radiation burn to the skin caused by too much exposure to
the sun’s UV rays or artificial sources such as tanning beds. Chronic sun
exposure creates premature cutaneous aging, decreases immune response to
environmental pathogens and increases the risk for developing premalignant and
malignant neoplasms [52-77]. The biggest risk factors for sunburn are the
amount of time the skin is exposed to UV rays, plus the intensity. Many factors
such as time of day, medications, ozone depletion, high altitude, clear skies
and skin phototypes influence sunburns [11]. Exposure to solar radiation has
the beneficial effects of stimulating the cutaneous synthesis of vitamin D and
providing radiant warmth. Unfortunately, when the skin is subjected to
excessive radiation in the ultraviolet range, deleterious effects may occur.
The most conspicuous is acute sunburn or solar erythema. Initially, UVR causes
vasodilation of cutaneous blood vessels, resulting in the characteristic
erythema. Within 1 h of UVR exposure, mast cells release preformed mediators
including histamine, serotonin and tumor necrosis factor, leading to
prostaglandin and leukotriene synthesis. Within 2 h after UV exposure, damage
to epidermal skin cells is seen. Erythema usually occurs 3-4 h after exposure,
with peak levels at 24 h [12]. Sunburns are graded as pink, red, and
blistering. In contrast, thermal burns are graded by degree (first, second and
third), but this classification should not be applied to sunburns because
thermal burns have quite different sequelae, such as scarring and death, which
are extremely rare consequences of sunburn. Keratoconjunctivitis or ocular
sunburn can also be caused by UV radiation, and it follows a similar time
course [13]. Studies have shown that UVA impairs the antigen presenting cell
(APC) activity of the epidermal cells and thereby causes immune suppression,
thus contributing to the growth of skin cancer [30]. In summary, UVA radiation
can cause nuclear and mitochondrial DNA damage, gene mutations and skin cancer,
dysregulation of enzymatic chain reactions, immune suppression, lipid
peroxidation (membrane damage) and photoallergic and phototoxic effects.
On the molecular level, exposure to UV radiation can result in a covalent
joining of pyrimidine (usually thymine) dimers. DNA repair mechanisms, such as
nucleotide excision repair, base excision repair, or mismatch repair genes, do
not recognize dimers; the mutations go uncorrected to the cell cycle. When
mutated genes reach the cell cycle, if not repaired by the induction of the p53
pathway, a series of changes can result in malignant transformation and
immunosupression. UV-induced immunosuppression contributes to skin cancer due
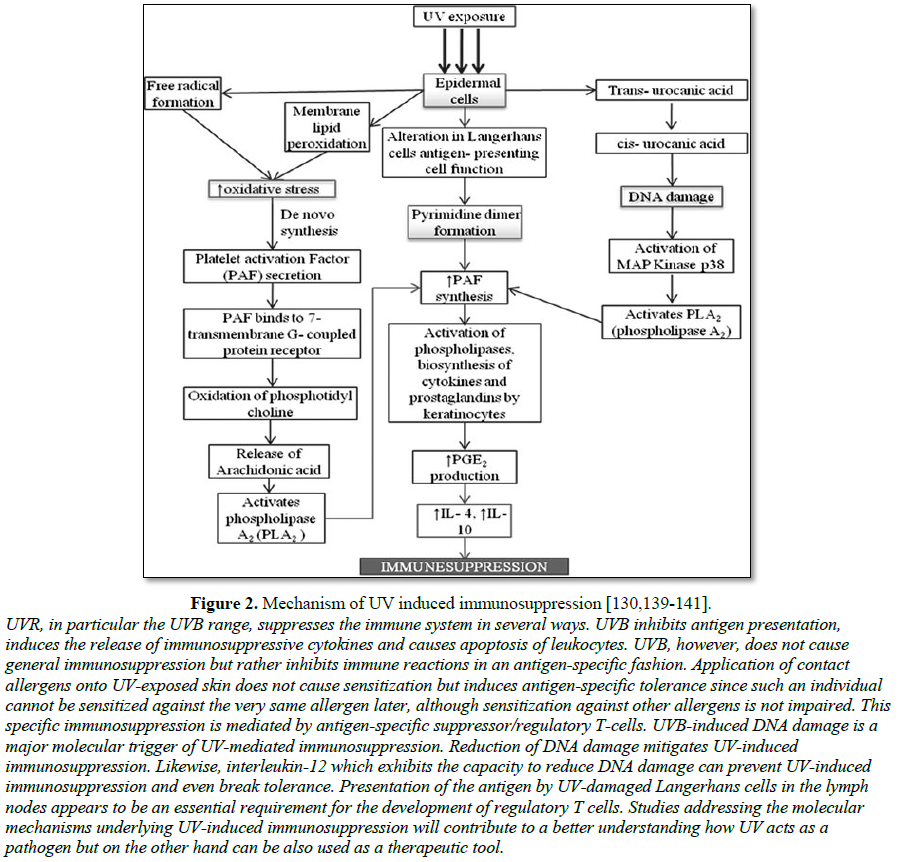
to damage to DNA and inhibition of protective mechanism within the skin (Figure
2). A common type of sun-related skin damage is actinic keratosis (AK).
Age, Fitzpatrick skin type 1 or 2 (Exhibit 1), and UV light are the
major risk factors for developing AK. Most AKs do not progress into invasive squamous
cell carcinoma (SCC), but the risk is still present. The risk of malignant
transformation of an AK to SCC within one year is approximately 1 in 1,000.
However, approximately 60% of invasive SCCs of the skin probably arise from
AKs. If not treated or protected against additional sun damage, AKs may
eventually progress to invasive SCC. Avoiding sun exposure and daily
application of sunscreen statistically decreases the number of AKs [77].
The diagnosis of SCC has increased over the past 30 years. The most
important risk factor for SCC is cumulative sun damage and age; the risk was
greatest in those with more than 30,000 hours of cumulative lifetime sun
exposure. UVA, UVB, PUVA and tanning beds have been shown to increase the
incidence of cutaneous SCC. Prevention of SCC includes protection from the sun,
including the use of protective clothing and application of sunscreen. Basal
cell carcinoma (BCC) is the most common skin cancer and occurs most frequently
on the face and head. In Caucasians, the incidence of BCC has steadily
increased and the lifetime risk of developing BCC is 30% [78-86]. BCC arises
from the basal layer of epidermis and its appendages. The most important risk
factor is chronic UVR. Other known risk factors include fair skin, light eyes,
red hair, chronic arsenic exposure, therapeutic radiation, immunosuppression,
basal cell nevus syndrome and various other genetic pre-dispositions. Primary
prevention is protection from sun exposure beginning at an early age [77].
Other than UVR, exposure to arsenic, radiation, chronic inflammatory conditions
in skin and burns, scars, infections complications or even tattoos are also the
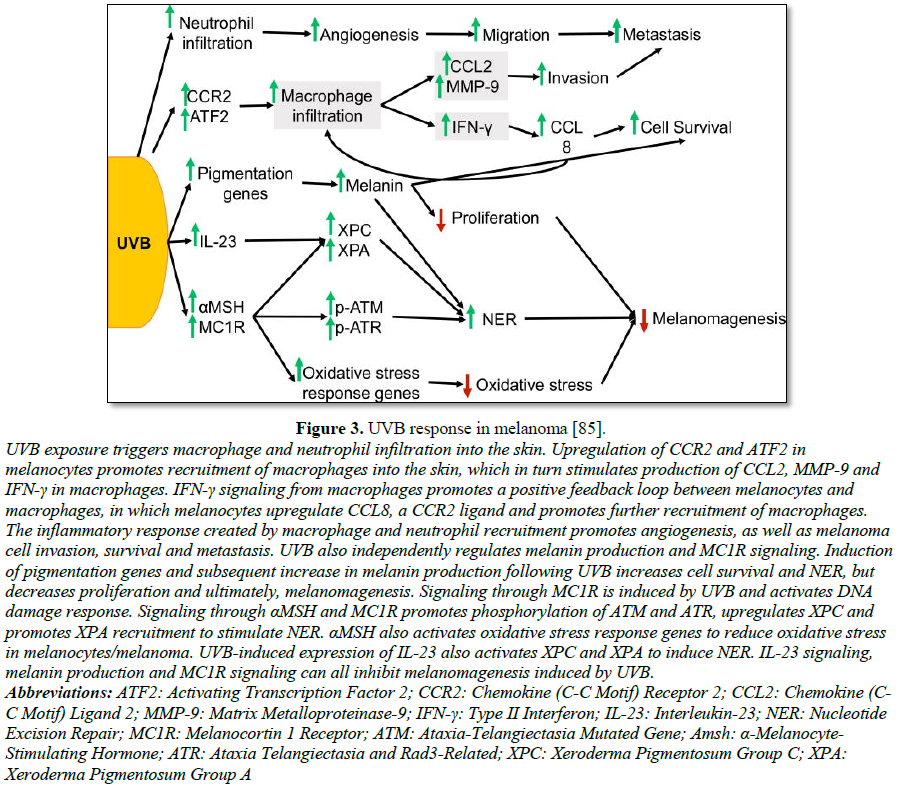
contributing factors for BCC [87-96] (Figure 3).
Epidemiological analysis reveals that annually in the United States
alone, ~5 million skin cancer patients are treated, and that between 40 and 50%
of Americans are susceptible to develop skin cancer at least once by the age of
65 [96]. More than 90% of melanoma cases in the United States are attributed to
skin cell damage from UV radiation exposure. Skin cancer is the most common
form of cancer, representing 40-50% of all cancers diagnosed in the US.
Melanoma is the most serious form of skin cancer. Melanoma is responsible for
the most skin cancer deaths, with about 9,000 persons dying from it each year
[84]. Despite early screening and detection programs, the overall mortality
rate from melanoma has remained stable or continues to rise. The incidence of
melanoma has more than tripled in the Caucasian population in the United States
over the past 30 years. An estimated 60-70% of cutaneous malignant melanomas
are thought to be caused by UV radiation exposure. Two types of UV radiation
are primarily responsible for causing carcinogenic skin damage: UVA (315 nm-400
nm) and UVB (280 nm-315 nm) [85]. Intense and intermittent sun exposure at a
young age increases a patient's risk of melanoma. Individuals with five or more
severe sunburns in childhood or adolescence have an estimated twofold greater
risk of developing melanoma. Melanoma is commonly found on areas sporadically
exposed to UVR, such as the back of the legs in women and the backs of men.
UVB, UVA and PUVA therapy all have been proven to increase the risk of
melanoma. Appropriate UVR protection decreases the risk of developing a
melanoma or having a secondary melanoma. Studies indicate that decreasing
recreational sun exposure following the diagnosis of primary melanoma can
significantly decrease the chance of developing a second melanoma [77] (Exhibit
2).
Photoaging
Every organ experience aging in various ways. Skin aging can be
categorized into three groups, intrinsic, photo and hormonal aging. The
consequences of photo-aging are usually characterized by morphological changes
including wrinkle formation or by histological changes in connective tissues.
In order to generate more wrinkles, vasculature structures are needed to
develop. Numerous reports suggested that angiogenesis plays a significant role
in inducing wrinkle formation in photo-damaged skin [97-142]. Among harmful
environmental factors that contribute to extrinsic aging, long-term effects of
repeated exposure to ultraviolet light are the most significant and are
referred to as photoaging. Premature skin aging and development of malignant
cutaneous tumors, melanoma and non-melanoma, are interrelated issues that are
increasingly important problems in the field of dermatology. Skin aging is
important aesthetically, whereas skin cancer is a direct threat to the health
of the patient [143]. Photoaging is a multisystem degenerative process that
involves the skin and skin support system. It is a cumulative process and
depends primarily on the degree of sun exposure and skin pigment. The epidermis
and dermis are both affected by UVB, but the dermis is also affected to a
significant extent by UVA. It has long been thought that the majority of human
photo-lesions due to UVB rays, now it is believed that UVA play a substantial
role in photoaging. Photoaging affects the sun-exposed areas and is
characterized clinically by fine and coarse wrinkling, roughness, dryness,
laxity, teleangiectasia, loss of tensile strength and pigmentary changes [144].
These changes are more severe in individuals with fair skin and are further
influenced by individual ethnicity and genetics. Photoaging may be prevented
and treated with a variety of modalities, including topical retinoids,
cosmeceuticals, chemical peels, injectable neuromodulators, soft tissue
fillers, and light sources [145]. There is also an increase in development of
benign and malignant neoplasms on photoaged skin. During the years the progress
has been made in understanding the photoaging in human skin. UV irradiation
invokes a complex sequence of specific molecular responses that damage skin
connective tissue [146]. In severely damaged skin, there is loss of epidermal
polarity (orderly maturation) and individual keratinocytes may show atypia,
especially the lower epidermal layers. More profound changes occur in the
dermis, where photodamage is characterized by degeneration of collagen and
deposition of abnormal elastic material, reflected by wrinkles, furrows, and
yellow discoloration of the skin. The greater the photodamage, the more the
accumulation of thickened, tangled and degraded elastic fibers [147-153]. The
application of topical growth factors after microneedling can be useful to
reduce visual signs of facial photoaging by improving skin texture and
minimizing the appearance of fine lines and wrinkles [151]. Amongst retinoids,
tretinoin is the most potent and best-studied retinoid. However, its irritation
potential has prompted dermatologists to switch over to less irritating but
comparably effective retinoids like adapalene and to some extent retinol and retinaldehyde
[154]. Bakuchiol (functional analogue of topical retinoids, as both compounds
have been shown to induce similar gene expression in the skin) is a
phytochemical that has demonstrated cutaneous anti-ageing effects when applied
topically. Bakuchiol is promising as a more tolerable alternative to retinol
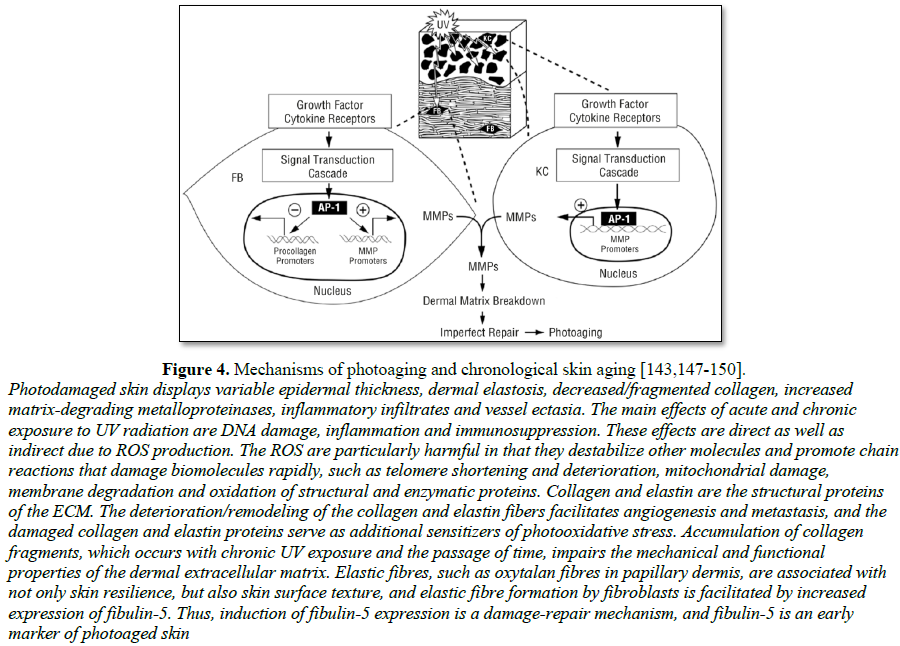
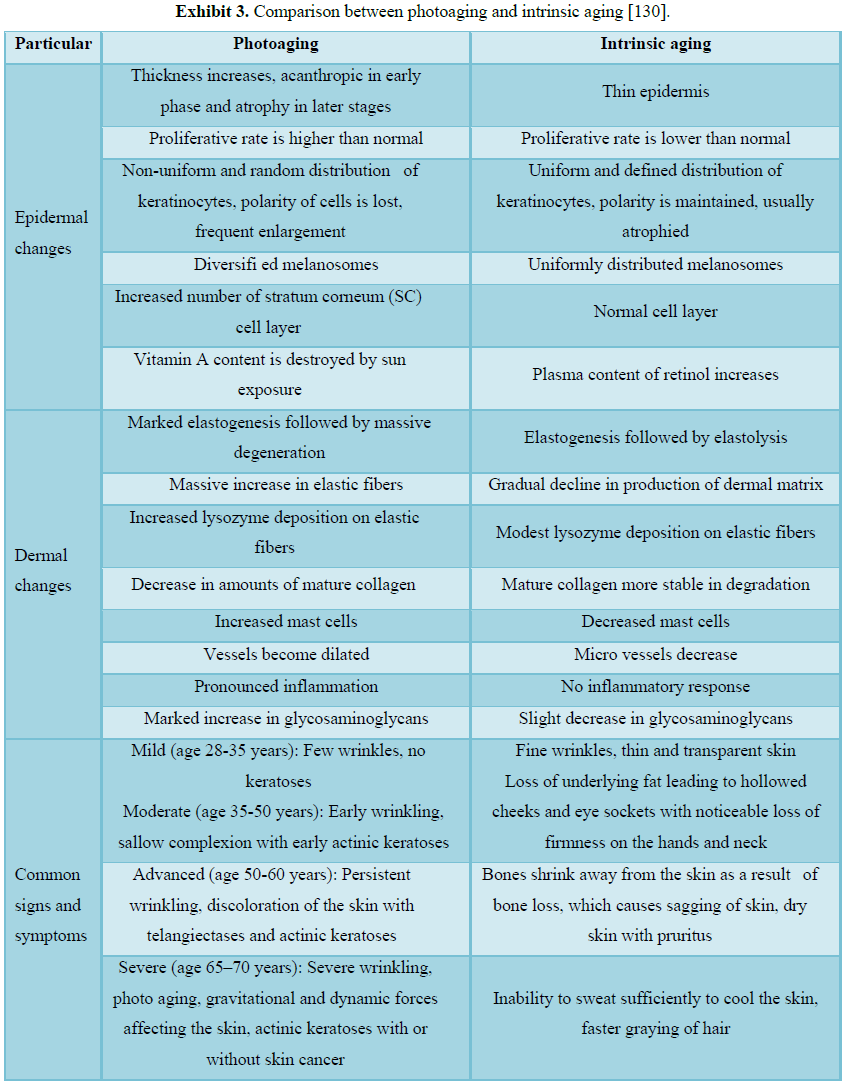
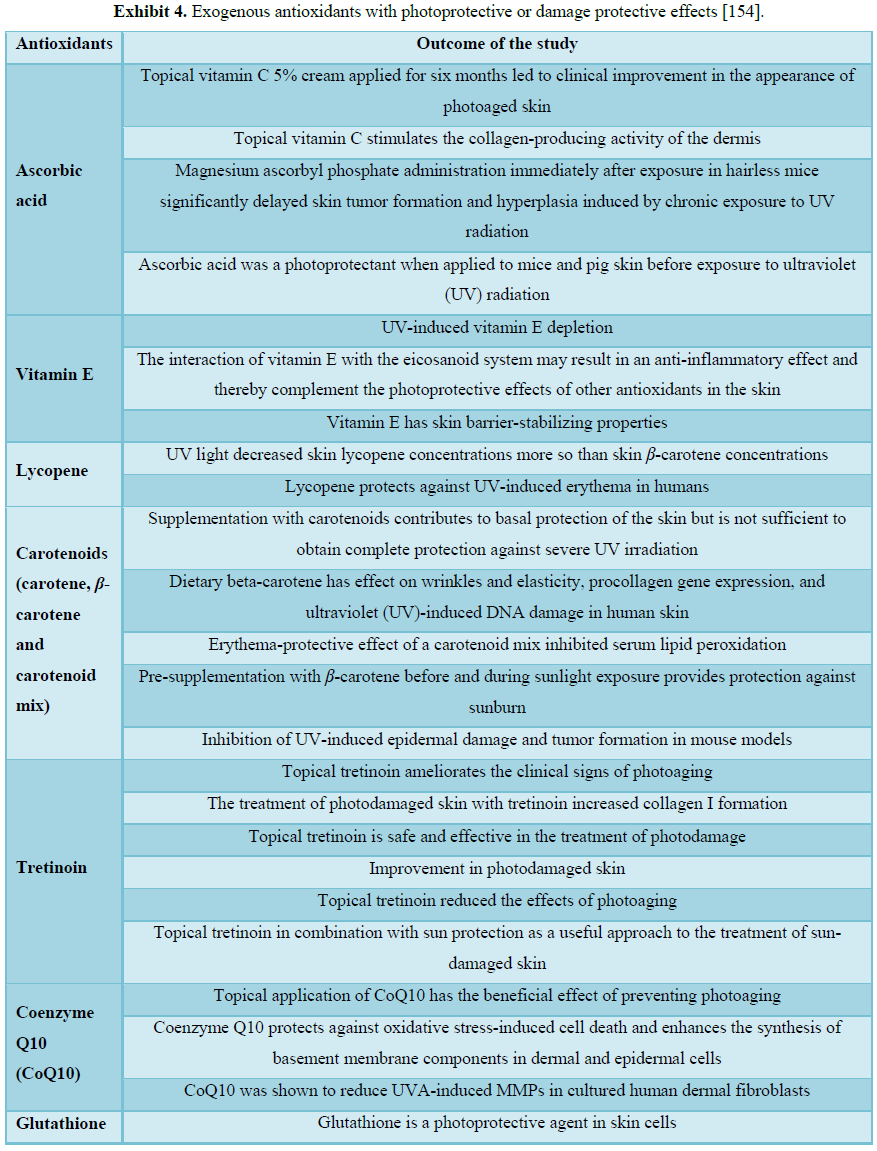
[152] (Figure 4 and Exhibits 3 and 4).
Sunburn home remedies and treatment
Certain medical treatments have been tried
and studied to treat sunburn. However, in general, most remedies have not shown
any clinically proven benefit as far as speeding the recovery or reversing the
damage. Therefore, most of the treatments available are only used to treat
symptoms.
·
Non-steroidal
anti-inflammatory drugs (NSAIDs): Systemic and topical non-steroidal
anti-inflammatory drugs, when used at dosages to achieve optimal serum levels
for anti-inflammatory effect, only result in an early and mild reduction of
ultraviolet B-induced erythema [15]. In oral (ibuprofen, Motrin, Naprosyn,
Advil, etc.) or topical diclofenac 0.1% gel (Solaraze) forms have shown to
reduce redness if applied before or immediately after UVB exposure. This
benefit may be diminished after 24 h. These medications may also help relieve
the symptoms of sunburn such as pain and discomfort [4].
·
Topical steroid creams have not shown any significant improvement in
sunburn symptoms. Oral steroids such as prednisone have not proven to be
beneficial and have been associated with some significant side effects.
Treatment with topical moderate-potency or high-potency corticosteroids does
not provide a clinically useful decrease in the acute sunburn reaction when
applied 6 or 23 h after UV exposure [16].
·
Applying Aloe vera gel: Topical application of Aloe vera is not an effective prevention
for radiation-induced injuries and has no sunburn or suntan protection [17].
The Aloe vera cream was continuing
applied at the test sites twice daily for the next three weeks. The results
showed no sunburn or suntan protection and no efficacy in sunburn treatment
when compared to placebo. The Aloe vera
cream has no bleaching effect too. However, this may be beneficial in treating
the symptoms [18].
·
Topical
anesthetics:
Advertised remedies such as topical anesthetics (benzocaine) may help with
symptoms of sunburn; however, very little clinical data is available to
substantiate their effectiveness. LMX is a topical liposomal formulation
containing 4% or 5% lidocaine. LMX 4% is available OTC and is FDA-approved for
temporary relief of pain and itching associated with minor cuts, minor burns,
sunburn, and insect bites [19].
·
Vitamin D
preparations:
Vitamin D enables anti-inflammation to promote tissue repair in response to
injury. Mechanistically, vitamin D signaling activated M2-autophagy regulators
Kruppel like factor 4 (KLF4), peroxisome proliferator activated receptor gamma
(PPARG) and arginase 1. Analysis of UV-exposed human skin biopsies detected a
similar increase in macrophage autophagy following vitamin D intervention,
identifying an essential role for autophagy in vitamin D-mediated protection of
skin from UV damage [25].
·
Sunscreens: Sunscreens represent a
practical approach to photoprotection for skin. The importance of beginning sun
protection at a young age cannot be overstated. In humans, the regular use of
sunscreens has been shown to reduce AKs, solar elastosis, UV-induced
immunosupression and photosensitivities. Sunscreens also prevent the formation
of SCCs in animals. A thorough understanding of the mechanism of action of
sunscreens, different sunscreen vehicle choices, and adverse effects can help
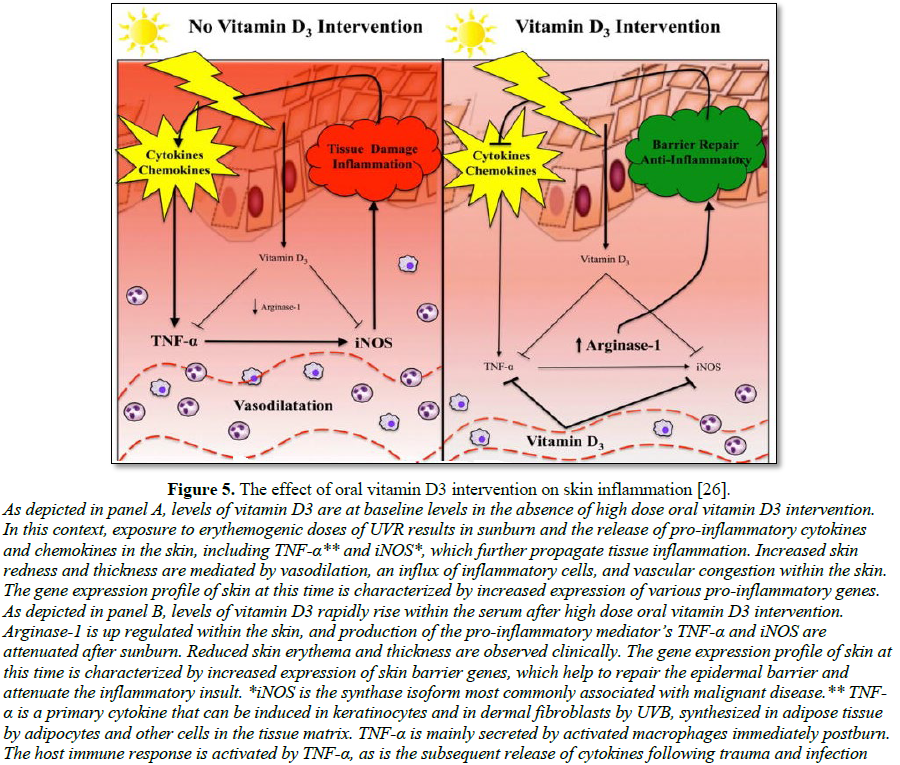
educate patients on their choice of sunscreens [77] (Figure 5).
Medication
responsible for increase skin sensitivity to sunlight
A large number of medications are known to increase skin sensitivity to
sunlight and are called photosensitive drugs or medications. Photo-sensitizing
chemicals usually have a low molecular weight (200 to 500 Da and are planar,
tricyclic or polycyclic configurations, often with heteroatoms in their
structures enabling resonance stabilization. All absorb UV and/or visible
radiation, a characteristic that is essential for the chemical to be regarded
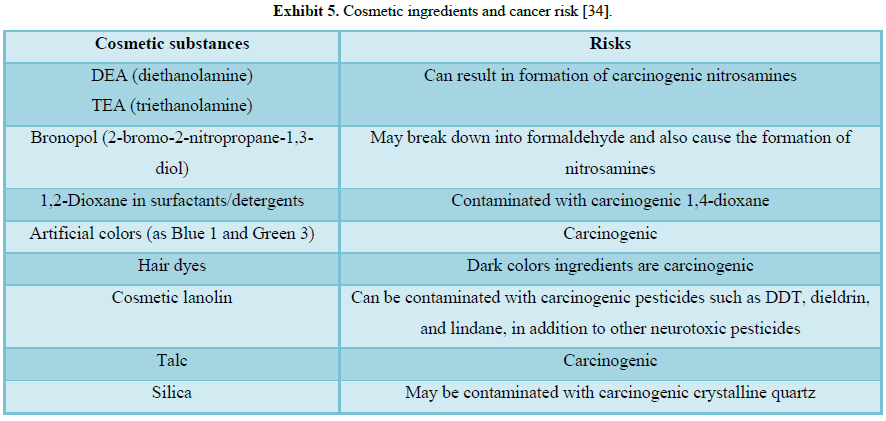
as a photosensitizer. Some of the common ones include (Figure 6 and Exhibits
5 and 6):
·
NSAIDs (Non-steroidal anti-inflammatory drugs)
·
Antibiotics: Tetracyclines
(tetracycline, doxycycline (Vibramycin)), Quinolone (ciprofloxacin (Cipro),
levofloxacin (Levaquin)), Sulfonamides (sulfamethoxazole and trimethoprim;
cotrimoxazole (Bactrim, Septra), sulfamethoxazole (Gantanol)).
·
Diuretics (water
pills): Thiazides
(hydrochlorothiazide (Hydrodiuril), furosemide (Lasix))
·
Cardiac
medications:
Amiodarone (Cordarone), quinidine
·
Diabetes drugs: Sulfonylureas such as
chlorpropamide (Diabinese), glyburide (Micronase, DiaBeta, Glynase)
·
Psychiatric
drugs: Chlorpromazine (Thorazine),
tricyclic antidepressants such as desipramine (Norpramin) and imipramine
(Tofranil)
·
Acne
medications: Isotretinoin
(Accutane) [20-24,34].
Tanning/pigmentation
with or without sun exposure
Some Africans and Asians avoid sun and use bleaching products to lighten
skin, while many Caucasians seek the sun for tanning to achieve a bronze skin
to “look good.” UV radiation from the sun or from artificial sources increases
skin pigmentation. Sunlight and indoor UV induced tanning is a common behavior,
especially among adolescents, young adults and individuals with lighter skin.
Several health benefit claims such as improved appearance, enhanced mood and
increased vitamin D levels have been attributed to tanning. The Indoor Tanning
Association claims that a base tan can act as “the body’s natural protection
against sunburn. Sunless tanning products may serve as a sensible, safer
alternative for those who desire tanned skin [155]. There are three phases of
tanning: immediate pigment darkening (IPD), persistent pigment darkening (PPD)
and delayed tanning (DT). IPD occurs during the first minutes of exposure to
UVA and then fades within few hours. PPD appears within hours of higher doses
of UVA exposure and persist up to several days or weeks. DT develops over 3-7 days
after UVB exposure and then remains for weeks. The mechanisms of UVA- and
UVB-induced pigmentation are different. UVA induces IPD and PPD through
oxidation of pre-existing melanin or melanogenic precursors. IPD is oxygen
dependent, and reactive oxygen radicals are considered to be responsible for
this process. PPD is also due to the upward movement of melanosomes toward the
surface of the skin. Persons with lightest skin (skin type I) do almost not
tan, while IPD and PPD are strongest in moderately and darkly pigmented skin.
DT results from synthesis of melanin in the melanocytes, followed by melanin
distribution to neighboring keratinocytes [156]. UVA (320-400 nm) causes
immediate pigment darkening (IPD) as well as persistent pigment darkening (PPD)
of skin within hours via photooxidation and/or polymerization of existing
melanin or melanogenic precursors due to the generation of reactive oxygen
species In contrast, UVB (280-320 nm) induces a slower but more stable type of
pigmentation termed delayed tanning (DT) which requires the increased synthesis
of melanin following the stimulation of tyrosinase activity and the entire
melanogenic cascade [157]. UVB-induced DT is photoprotective (it is estimated
to have a SPF of 3) while DT induced by UVA is not considered to be
photoprotective. DT is maximal from 10 days to 3-4 weeks, depending on the UV
dose and the individual's skin color. It may take several weeks or months for
the skin to return to its base constitutive color. UVA-induced DT is 2-3 orders
of magnitude less efficient per unit dose than UVB and has an earlier onset,
often directly after IPD [158]. The IPD method can also be used as an
appropriate endpoint in the determination of UVA protection. It is time saving,
and thus considerably lowers the risk of UV exposure, particularly when testing
sunscreen products with higher UVAPF [159]. IPD typically appears grey to black
while PPD is brown. Both IPD and PPD do not require any new pigment synthesis
and are thought to result from oxidation and/or polymerization of pre-existing
melanin or melanogenic precursors and metabolites. Melanosomes have also been
shown to redistribute within both keratinocytes and melanocytes during the
IPD/PPD response. PPD is protective against neither UVB-induced erythema nor UVB-induced
DNA lesions [160]. Exposure to ultraviolet radiation from indoor tanning device
use is associated with an increased risk of skin cancer, including risk of
malignant melanoma and is an urgent public health problem. By reducing indoor
tanning, future cases of skin cancer could be prevented, along with the
associated morbidity, mortality and healthcare costs (Figure 7 AND Exhibits
7 and 8) [161-175].
The sun care industry
The U.S. sun care market size was estimated at USD 1.95 billion in 2016.
The growing consumer awareness regarding the ill-effects of over exposure to
ultraviolet (UV) rays on the undefended skin is expected to propel growth.
Furthermore, rising utilization of organic sun care products owing to absence
of synthetic chemicals in the formulation is expected to drive growth over the
forecast period. The sunscreen manufacturers are actively using these
formulations as it increases the endurance of skin cells against UV rays and
enhances the self-defence mechanism. Enhanced standard of living coupled with
increasing disposable income of the middle-class working population in
economies of North America, including the U.S., is expected to propel sun care
market growth over the forecast period. The industry players including L’Oréal
are using nanotechnology by manipulating the materials at an atomic or
molecular in order to enhance the efficiency of the product. Over the past five
years, the Sunscreen Manufacturing industry has grown by 1.6% to reach revenue
of $407m in 2018. In the same timeframe, the number of businesses has grown by
3.9% and the number of employees has grown by 1.5% [178-180].
CLASSIFICATION OF
SUNSCREENS
Sunscreens are classically divided into physical or chemical sunscreens.
Chemical sunscreen absorbs into the skin and then absorbs UV rays, converts the
rays into heat and releases them from the body. Physical blockers are inorganic
and reflect, scatter and/or absorb UVR. Physical UV absorbers like titanium
dioxide and zinc oxide have been considered as highly protective agents against
UVR. ZnO and TiO2 combinations are particularly valuable because of
their ability to filter both UVA and UVB radiations, providing broader UVR
protection than that observed with individual component [95]. They are used in
sunscreens as nanoparticles, which denotes a size <100 nm. The smaller size
of these mineral particles increases their cosmetic acceptability by users as
they are much less visible after application. ZnO has a broad UVA-UVB
absorption curve, while TiO2 provides better UVB protection.
Overall, the human health risks with inorganic filters are extremely low given
a lack of percutaneous absorption; however, there is potential risk when
exposed via inhalation, prompting recommendations against spray sunscreen
products with nanoparticles [111]. UV-A rays heavily contribute to both
premature skin aging and skin cancer, while UV-B rays cause sunburn. Hence, the
use of sunscreen is strongly encouraged by many healthcare practitioners in
order to minimize or possibly eradicate the harmful effects of UV rays on our
skin, keeping in mind, that about 90% of all skin cancers are associated with
exposure to the sun’s harmful radiation. Protection against both UVB and UVA
radiation is advocated. Most sunscreens combine chemical UV absorbing sunscreens
and physical inorganic sunscreens, which reflect UV, to provide broad-spectrum
protection. The correct use of sunscreens should be combined with the avoidance
of midday sun and the wearing of protective clothing and glasses, as part of an
overall sun protection regimen [94,95]. Unfortunately, inaccurate information
is currently roaming the media and the Internet regarding the safety, toxicity,
and acute side effects of the active ingredients currently used in sunscreens,
therefore discouraging people from using sunscreens [92]. Chemical sunscreens
are organic and generally aromatic compounds conjugated with a carbonyl group.
They are designed to absorb high-intensity UVR, produce excitation to a higher
energy state, and, with the return to the ground state, result in conversion of
the absorbed energy into a longer, lower energy wavelength. Chemical sunscreens
can be classified based on their portion of UV coverage. Commonly known
ingredients for UVB sunscreen protection are Padimate O, octinoxate, octisalate,
octocrylene and ensulizole. Commonly known Studies reveal that Persistent
Pigment Darkening (PPD) to be a stable end point inducible by all the UVA
wavelengths, not affected by fluence rate, i.e., a reliable endogenous UVA
dosimeter in the skin [89]. Problems associated with chemical sunscreens are:
(i) Requires about 20 min after application before it starts to work; (ii)
Chemical sunscreens may cause side effects, such as erythema, edema and
irritation (especially for those who have dry skin with a damaged moisture
barrier) due to the multiple ingredients combined in order to achieve broad
spectrum UVA and UVB protection; (iii) The higher the SPF (such as formulas of
SPF 50 or greater), the higher the risk of irritation for sensitive skin types;
(iv) The protection it offers gets used up more quickly when in direct UV
light, so re-application must be more frequent. (v) Increased chance of redness
for rosacea-prone skin types because it changes UV rays into heat which can
exacerbate flushing; (vi) May clog pores for oily skin types [93]. The
importance of adequate UVA protection has become apparent in recent years. The
United States and Europe have different standards for assessing UVA protection
in sunscreen products. The majority of tested sunscreens offered adequate UVA
protection according to US Food and Drug Administration guidelines for
broad-spectrum status, but almost half of the sunscreens tested did not pass
standards set in the European Union. UVA sunscreen ingredients are oxybenzone, meradimate,
avobenzene and tetraphthalydine dicamphor sulfonic acid [90,91]. Broad spectrum
is a term designed to mean protection from both UVA and UVB. Problems
associated with physical sunscreens are: (i) Can rub off, sweat off and rinse
off easily, meaning more frequent re-application when outdoors as needed; (ii)
The physical sunscreen agents impart high opacity to the topical preparation,
which makes the formulated creams cosmetically unacceptable and leave a whitish
layer on the skin, making some formulas incompatible for medium to dark skin
tones; (iii) Can be less protective if not applied and re-applied generously
and accurately since UV light can get between the sunscreen molecules and get
into the skin [93]. There are few reports available on the application of
Polymeric Nanoparticles (NPs) of physical and chemical sunscreen ingredients
(titanium dioxide, zinc oxide, octyl methoxycinnamate, oxybenzone, octocrylene
and luteolinon) to improve sun protection efficacy. UV-absorbing agents must
accumulate within the upper skin layers in order to provide a dense
light-absorbing layer and guarantee water resistance. Incorporation of
antioxidants could provide additional benefit by scavenging free radicals.
Natural polyphenols are attractive in this respect, due to their potential
activity as photoprotectans and antioxidants [95].
FDA APPROVED
SUNSCREEN INGREDIENTS: LITERATURE REVIEW
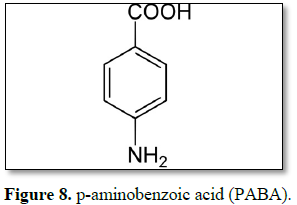
Aminobenzoic acid
Aminobenzoic acid is a Vitamin B Complex Member. The chemical
classification of aminobenzoic acid is Vitamin B Complex Compounds. In 1943,
p-aminobenzoic acid (PABA) was patented. Red petrolatum was used by the United
States military as a sunblock during World War II. The initial compounds were
primarily ultraviolet B radiation (UVB) blockers as it was to blame for the
most observable effect, sunburn [48]. When exposed to light, aminobenzoic acid
(para-aminobenzoic acid or PABA) absorbs UV light and emits excess energy via a
photochemical reaction that may cause damage to DNA. Because DNA defects
contribute to skin cancer, aminobenzoic acid is no longer widely used in
sunscreen formulations. Aminobenzoic acid may also increase oxygen uptake at
the tissue level and may enhance monoamine oxidase (MAO) activity to promote
the degradation of serotonin, which in excess, may lead to fibrotic changes
[46]. Aminobenzoates are the most potent UVB absorber but do not absorb UVA.
Their use has declined due to para-aminobenzoic acid (PABA) sensitivity. PABA
is a very effective UVB filter; however, it was reportedly the most common
photoallergen and contact allergen (Figure 8) [47].
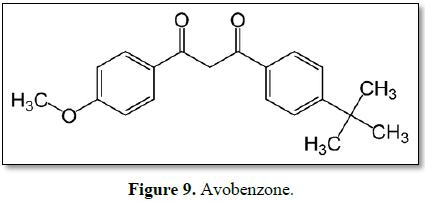
Avobenzone
UVA blockers, considered broad spectrum and have a high efficacy against
UVA I (>380 nm); however, they are very photounstable and lose from 50% to
90% of their particles after 1 h of UV exposure. It is also reported that they
degrade the UV filter octinoxate [40,59]. Avobenzone is the most widely used
UVA filter in sunscreen lotion and it is prone to degradation in the presence
of sunlight/UV radiation, they show a significant skin penetration [56]. 30%
HPCD-Avobenzone system was the most photostable following UVA exposure at
various regimens and also enabled photoprotective efficiency in vivo, evidenced by lower levels of
sunburn cells and skin edema induction [60]. To overcome the photo-instability
of avobenzone, various photostabilizers have been used as additives, including
antioxidants such as vitamin C, vitamin E and ubiquinone. The dual role of
glutathione as a skin whitening agent and a photostabilizer of avobenzone may
be useful for the development of multipurpose cosmetic lotions [49]. Avobenzone
is dibenzoyl methane derivative. It is oil soluble crystalline solid [50,58].
Mesoporous silicas (MSs) containing avobenzone or oxybenzone effectively
ameliorated UVA-induced skin disruption and reduced the possible toxicity
elicited by percutaneous penetration [56]. Photosensitization by drugs is a
problem of increasing importance in modern life. This phenomenon occurs when a
chemical substance in the skin is exposed to sunlight. Photosensitizing drugs
are reported to cause severe skin dermatitis, and indeed, it is generally
advised to avoid sunbathing and to apply sunscreen. In this context, the NSAID
diclofenac is a photosensitive drug, especially when administered in topical
form. avobenzone provides partial photoprotection to diclofenac from
photocyclization to carbazole derivatives [52]. Avobenzone is widely used in
various personal care products, is present in swimming pools, and is toxic to
aquatic organisms. Avobenzone induces mitochondrial dysfunction-mediated
apoptosis leading to abnormal placentation during early pregnancy [53].
Avobenzone was more unstable/toxic than octyl p-methoxycinnamate (an organic
UV-B filter originally developed in the 1950s, has been one of the most widely
used sunscreens for decades) [54,55]. Octocrylene helps stabilize avobenzone,
which is good, but it is a known endocrine disruptor that also releases free
radicals (Figure 9) [57].
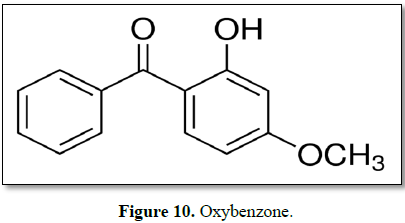
Oxybenzone
Oxybenzone is a benzophenone derivative used as a sunscreen agent.
Oxybenzone absorbs UVB and UVA II rays, resulting in a photochemical excitation
and absorption of energy [116]. Oxybenzone (Benzophenone-3) is an emerging
human and environmental contaminant used in sunscreens and personal care
products to help minimize the damaging effects of ultraviolet radiation. The
Center for Disease Control (CDC) fourth national report on human exposure to
environmental chemicals demonstrated that approximately 97% of the people
tested have oxybenzone present in their urine, and independent scientists have
reported various concentrations in waterways and fish worldwide. Oxybenzone can
also react with chlorine, producing hazardous by-products that can concentrate
in swimming pools and wastewater treatment plants. Moreover, adverse reactions
could very well be increased by the closed loop of ingesting fish contaminated
with oxybenzone and/or washing the ingredient off our bodies and having it
return in drinking water as treatment plants do not effectively remove the
chemical as part of their processing protocols. In humans, oxybenzone has been
reported to produce contact and photocontact allergy reactions, implemented as
a possible endocrine disruptor and has been linked to Hirschsprung's disease.
Environmentally, oxybenzone has been shown to produce a variety of toxic
reactions in coral and fish ranging from reef bleaching to mortality (Figure
10) [117].
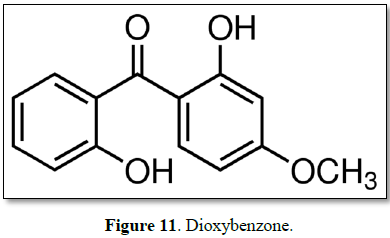
Dioxybenzone
Dioxybenzone is an approved sunscreen ingredient in concentrations up to
3% [67]. The benzophenone sunscreens, octabenzone (UV-1) and dioxybenzone
(UV-2) were found to exhibit significant chemopreventive activity against mouse
skin carcinogenesis which correlated with their antioxidant potency [63]. In a
study aimed to qualify photo-safety screening, benzophenone derivatives and
ketoprofen exhibited significant reactive oxygen species (ROS) generation upon
exposure to simulated sunlight; however ROS generation from sulisobenzone and
dioxybenzone was negligible [68]. Polymerization with natural polymer pullulan
not only provides a long polymer backbone to dioxybenzone, but also keeps the
distance between benzene rings of the dioxybenzone and prevents reduction of
photoabsorption intensity. UV/Vis spectrophotometry confirmed that
dioxybenzone-pullulan polymer and dioxybenzone demonstrated similar UV
absorption. Dioxybenzone showed higher plasma concentration after multiple
applications compared to that of dioxybenzone-pullulan polymer [64]. Chlorine
is used as a chemical disinfectant in swimming pools. Its reactivity suggests
sunscreen components might be chlorinated, altering their absorptive and/or
cytotoxic properties. Chlorinated oxybenzone and dioxybenzone (caused
significantly more cell death than unchlorinated controls. In contrast,
chlorination of sulisobenzone actually reduced cytotoxicity of the parent
compound. Exposing a commercially available sunscreen product to chlorine also
resulted in decreased UV absorbance, loss of UV protection, and enhanced
cytotoxicity [65]. Bromal hydrate was also detected as one of the byproducts
generated by oxybenzone and dioxybenzone, which is highly toxic; the “positive
halogen” properties of bromal hydrate are responsible for its high toxicity
[66]. In addition to allergic reactions, concerns have been raised about the
relative ease of which benzophenone is absorbed into the skin and may promote
generation of potentially harmful free radicals (Figure 11) [69].
Cinoxate
Ester derivatives such
as ethylhexyl methoxycinnamate (octinoxate), isoamyl p-methoxycinnamte
(amiloxiate), octocrylene and cinoxate are used in cosmetics all over the world
as UV filters. However, their maximum concentrations in cosmetic products are
restricted due to their adverse effects, which include contact and a
photocontact allergies, phototoxic contact dermatitis, contact dermatitis,
estrogenic modulation and generation of reactive oxygen species.
4-hydroxycinnamic acid, which is currently indexed as a skin-conditioning
cosmetics ingredient, has been widely tested in vitro and in vivo as a
new drug candidate for the treatment of hyperpigmentation [61]. Cinnamates have
replaced PABA as the next most potent UVB absorber and include octinoxate (OMC)
and cinoxate (2-ethoxyethyl-methoxycinnamate). Cinoxate is less commonly used
[62]. Major Key players with cinoxate are Major Key Players are Parchem (US),
Carbosynth Limited (UK), Synchem UG & Co. KG (DE) (Figure 12) [110].
Octinoxate
Octinoxate (Octyl
Methoxycinnamate, OMC) is a cinnamate ester and common ingredient in sunscreen
and other skin care products to minimize DNA photodamage. It was originally
developed in 1950's as an organic UV-B filter that absorbs UV-B rays from sun.
It is often combined with nanoparticles or other water-resistant liposomes in
formulations to increase the localization at the epidermis and decrease the
risk of percutaneous absorption [105]. OMC is the most commonly used UVB filter
in the US. It is not as potent a UVB absorber as padimate O; for this reason,
other UVB absorbers are used in combination to increase the SPF. OMC is not
very photostable and degrades in the presence of sunlight after a short period
of time. OMC is a potent UVB absorber and is the most frequently used sunscreen
ingredient. The efficacy of OMC can be further increased when encapsulated in
polymethyl methacrylate microsphere [62]. Due to low water solubility (<1
mg/L), OMC is suitable for most waterproof sunscreen formulations [106]. Hawaii
legislature passed a bill that outlaws products that contain Oxybenzone and
OMC, effective January 2021. The bill is based on studies that show that
oxybenzone may harm coral larvae and that both compounds may “bleach” coral,
causing it to lose symbiotic algae [107-109]. OMC has been detected in human
urine, blood and breast milk, which indicates that humans are systemically
exposed to this compound. OMC is an endocrine disruptor that mimics estrogen
and can disrupt thyroid function (Figure 13) [112,113].
Homosalate
Homomenthyl salicylate or 3,3,5-Trimethylcyclohexyl Salicylate. After
topical application of gel, the bioavailability of HMS was 5.4 ± 1.1 and 4.2 ±
0.6% for high and low doses (10 and 20 mg), respectively. Homosalate (HMS) is
an UV filtering agent used in sunscreens and other cosmetics for skin
protection purposes. It is a viscous or light yellow to slightly tan liquid or
oil, derives from salicylic acid [70,71]. Salicylates are weak UVB absorbers
and they are generally used in combination with other UV filters. Both
octisalate and homosalate are water insoluble that leads to their high
substantivity, which is the ability to retain its effectiveness after exposure
to water and perspiration [72]. HMS does not have endocrine disruptor effects
on thyroid function and the pubertal development of female and male rats [73].
Another study says HMS is a potential endocrine disruptor and studies in cells
suggest it may impact hormones. In addition to direct health concerns following
homosalate exposure, the chemical may also enhance the absorption of pesticides
in the body [75]. Homosalate suppressed the ear edema response to dinitrobenzene;
a possible mechanism of action of the salicylates is suppression of COX (Figure
14) [114].
Octisalate
Also, Ethylhexyl salicylate, a benzoate ester and a member of phenols. It
derives from a salicylic acid. Salicylates are weaker UVB absorbers. It has an
approved usage level of 3 to 5% in both US and EU and is a good solubilizer for
benzophenone 3. In suntan lotions, it is used as a preservative and
antimicrobial. This salicylate salt is found in wintergreen leaves [115]. They
have a long history of use but were supplanted by the more efficient PABA and
cinnamate derivatives. They are generally used to augment other UVB absorbers.
With the trend to higher SPFs, more octisalate or octyl salicylate (ethylhexyl
salicylate) is being used followed by homosalate or homomenthyl salicylate.
Both materials have the ability to solubilize oxybenzone and avobenzone [97].
Both homosalate and octisalate were found to suppress experimental autoimmune
encephalomyelitis (EAE), a widely used animal model of Multiple sclerosis (MS).
Thus, salates may be useful new insight into mechanisms of controlling
autoimmune disease (Figure 15) [114].
Meradimate (menthyl
anthranilate)
Meradimate, before known as menthyl anthranilate, is used in a maximal
concentration of 5% in different products as a UV filter. It is currently
required to be named as meradimate in all FDA approved OTC products. Meradimate
is approved by the FDA and Health Canada to be used as an ingredient in sun
blocking products Meradimate is a monoterpenoid, a broad-spectrum ultraviolet
absorber used as a chemical filter in commercial sunscreens [76]. A moderately
effective UVA protector not permitted for use in Europe or Japan. Its
protective effective action does not cover completely the UVA rays as it only
reaches 336 nm. This has been proven even though meradimate has a theoretical
protective coverage range between 200-380 nm. Its function is related to the
intrinsic structure of meradimate which is an ortho-disubstituted
aminobenzoate. This structure allows easy electron delocalization and shifts in
the maximum absorption. Poor photoprotection reported by Rodrigues et al. [76]
makes it a poor choice for an efficient, efficacious sunscreen chemical filter (Figure 16).
Octocrylene
2-Ethylhexyl-2-cyano-3,3 diphenylacrylate or octocrylene is chemically
related to cinnamates. It can be used to boost SPF and improve water resistance
in a given formulation. Octocrylene is photostable and can improve the
photostability of other sunscreens. It is expensive and can present
difficulties in formulation [97]. The absorption profile of octocrylene spans
from 290 to 360 nm with peak absorption at 307 nm. The compound has an
excellent safety profile with low irritation, phototoxicity, and photoallergic
potential. Octocrylene may be used in combination with other UV absorbers to
achieve higher SPF formulas and to add stability. [62]. Photostability refers
to the ability of a molecule to remain intact with irradiation. Photostability
is potentially a problem with all UV filters. This issue been raised
specifically with avobenzone. This effect may degrade other sunscreens in a
formulation, including octyl methoxycinnamate. Octocrylene and some of the
newer sunscreens, including BEMT, stabilized avobenzone [97]. Chlorination
reactions showed that only octocrylene was stable in chlorinated seawater [66].
UVA and UVB screening potentials of octocrylene and zinc oxide formulations
were compared in the 290-400 nm wavelength region. Zinc oxide loaded SLN
suspensions were found to be more effective in the UVA region while octocrylene
loaded ones performed better in the UVB region [98]. Octocrylene appears to be
a strong allergen leading to contact dermatitis in children and mostly
photoallergic contact dermatitis in adults with an often-associated history of
photoallergy from ketoprofen. Patients with photoallergy from ketoprofen
frequently have positive photopatch test reactions to octocrylene. These
patients need to be informed of sunscreen products not containing octocrylene,
benzophenone-3, or fragrances [99,100]. The clinical studies show that
octocrylene is both a photocontact allergen and a contact allergen [103].
Octocrylene's ability to cause contact allergy is probably attributable to its
reactivity towards lysine [101]. A reduction of the UV filter in the
formulation packed in HDPE/LDPE material can occur over time, reducing the
protective effect of the product when applied to the skin [102]. Wide
application in cosmetics leads to contamination of the aquatic environment,
mainly affects transcription of genes related to developmental processes in the
brain and liver as well as metabolic processes in the liver (bioaccumulation
study in male zebrafish) (Figure 17) [104].
Padimate O
Padimate A and Padimate O were introduced as substitutes for PABA to
reduce the number of reactions seen with PABA. Ethylhexyl dimethyl PABA is also
known as padimate O, OD-PABA or octyl dimethyl p-aminobenzoate. It is a viscous
liquid tends to retain on the surface of the stratum corneum with little
penetration. It has a very low potential for sensitization. Ester derivatives
of PABA became more popular with greater compatibility in a variety of more
substantive vehicles and a lower potential for staining or adverse reactions.
Padimate O is an active sunscreen agent in cosmetics and over-the-counter
sunscreen drug products in concentrations up to 8%, as regulated by the FDA. No
irritation was reported when both eyes are washed and left unattended. 5%
ethylhexyl dimethyl PABA mixed with mineral oil applied to rabbit skin did not
cause irritation and no skin sensitization in guinea pigs with the same. High
concentrations of padimate O may be toxic to the epididymis and caution should
be exercised when administering this substance to infants younger than six
months of age due lack of understanding of its metabolism and absorption. It is
widely used as an ingredient in many cosmetics at an average concentration of
1.25% (0.5-2.0%) in Korea. It is toxic to the following four organs: testis,
epididymis, spleen, and liver. In addition, experiments using human
keratinocytes found that ethylhexyl dimethyl PABA inhibits cell growth and DNA
synthesis at low concentrations, and halted the cell cycle of malignant
melanoma cell line (MM96L) at the G1 phase. According to the EWG database, it
is used in many products including lipstick, conditioner, shampoo, anti-aging
agents, hair spray, and sunscreen. No significant reproductive toxicity,
genotoxicity, carcinogenicity, skin sensitization, skin irritation or
phototoxicity was observed in response to Padimate O administration (limited
studies there, requires further investigations) (Figure 18) [97,118,119].
Ensulizole
2-phenylbenzimidazole-5-sulfonic acid/ Phenylbenzimadazole sulfonic acid
or ensulizole is a water-soluble UVB absorber that can be used in the water
phase of emulsion systems. It is commonly found in cosmetic products and
sunscreen formulas in combination with other UV filter compounds due to its
minimal protection against UV-A wavelengths, indicated to be used as an
UV-B-absorbing molecule in sunscreen formulations. In contrast to most
oil-soluble sunscreen ingredients, allowing for a less-greasy, more
aesthetically pleasing formulation such as a daily use moisturizer containing
sunscreen. Phenybenzimidazole sulfonic acid boosts the SPF of organic and
inorganic sunscreens. It can also be used in clear gels owing to its water
solubility. Due to its water solubility, ensulizole is commonly used in
products formulated to feel light and less oily. It was demonstrated by studies
that ensulizole treatment provided protection against cyclobutane pyrimidine
dimers and photosensitized the formation of oxidized guanine bases after UV-A
or UV-B exposure. According to the FDA, the maximal approved concentration of
ensulizole is 148 mM although concentrations ranging between 74 and 148 mM can
be found in commercial sunscreen products. Ensulizole is capable of generating
reactive oxygen species, including singlet oxygen upon photoexcitation. Based
on the findings in vitro and in
cellulo, ensulizole induces damage on the DNA, causes DNA strand breaks and
photosensitizes the formation of oxidized guanines via type I and II
photosensitization mechanisms following UV-A or UV-B irradiation (Figure 19)
[87,120-125].
Sulisobenzone
A benzophenone does not undergo decomposition to an inactive form if it
does not couple to target molecules. Instead, it degrades from the
photo-excited state back to its initial state, so it can be once again
photolyzed to an active state. This process increases the likelihood that the
benzophenone will couple to a target molecule during the photoreaction.
Benzophenones are used as photoinitiators, fragrance enhancers, ultraviolet
curing agents, and, occasionally, as flavor ingredients; they are also used in
the manufacture of insecticides, agricultural chemicals, and pharmaceuticals
and as an additive for plastics, coatings, and adhesives. The UV-filter
substance Sulisobenzone (BP-4) is widely employed in sunscreens and other
personal care products. Sulisobenzone is approved by the FDA in concentrations
of up to 10% and in Canada, is approved by Health Canada at the same
concentrations. It works to filter out both UVA and UVB rays, protecting the
skin from sun UV damage. The benzophenones are a group of aromatic ketones that
have both pharmaceutical and industrial applications. Benzophenones may be
found organically in fruits such as grapes, used primarily as a UVA absorber,
but boosts SPF values in combination with other UVB absorbers. Sulisobenzone
water soluble, somewhat unstable, and used with less frequency. It can cause
skin and eye irritation. Does not penetrate the skin to a large degree, but
enhances the ability of other chemicals to penetrate. Safety use in sunscreen
is questionable (Figure 20) [88,126-130].
Trolamine salicylate
Trolamine salicylate is an organic compound or a salt formed between
triethanolamine and salicylic acid. Triethanolamine neutralizes the acidity of
the salicylic acid. It is a topical analgesic used for temporary relief of
minor pain associated with arthritis, simple backache, muscle strains, sprains,
and bruises. Unlike other topical analgesics, trolamine salicylate has no
distinct odor which improves patient acceptability. Trolamine or
triethanolamine salicylate has good water solubility. It is mostly used for
water soluble sunscreens to help increase the SPF of cosmetics owing to their
substantivity to the skin. It also displays low systemic absorption upon dermal
or topical administration and has low skin irritant properties. Approved in
both the US and Canada, trolamine salicylate is used in concentrations of up to
12%. It filters UVB rays, but has no effect on UVA rays, which means it cannot
be relied on alone as a sunscreen. It has found to cause photocontact dermatitis
(Figure 21) [106,131,132].
Titanium dioxide
TiO2 occurs naturally in three crystalline structures: rutile,
anatase and brokite. Rutile is the most common and stable form of this pigment.
Important optical properties of this birefringent crystal are its refractive
indices in the UV and visible wavelength range. The USP classifies it as a
topical protective. The protection is primarily for its opacity due to high
refractive index (2.7). However, the high refractive index is not the only
property that determines how well a substance blocks light. The film thickness
in which the substance is suspended as well as the size of the individual
particles also affects the efficacy of the sunscreen. Advantages offered by sunscreens
based on inorganic compounds comprise absence of skin irritation and
sensitization, inertness of the ingredients, limited skin penetration, and a
broad-spectrum protection. As a solar ray protective, it is used in a conc. 5%
to 25% in ointments and lotions. Since it is not absorbed into the skin, this
effect may not be an issue in topical use on unbroken skin. It is also used as
a white pigment in cosmetics and paints. According to the US FDA, the
protection factor against UVA should be at least one-third of the overall sun
protection factor. Previously, TiO2 had a suboptimal cosmetic
profile, appearing thick and white on application. Current formulations are
micronized or nanoparticle formulations, which blend in with the skin tone and
attain better cosmesis. As micro-sized TiO2 is the most effective in
UVB and micro-sized ZnO in the UVA range, the combination of the two oxides
assures the required broad band UV protection. The particle sizes that result,
within human SC, in higher UV absorption and scattering and lower UV
transmission improve the UV attenuation. Emulsions are generally unstable,
often change from water-oil to oil-water or break down during application on
the skin, and may increase the cutaneous permeability. It has been demonstrated
that sunscreen TiO2 particles penetrate deeper into human skin from
an oily than from an aqueous dispersion. The International Agency for Research
on Cancer (IARC) has recently classified TiO2 as an IARC group 2B
carcinogen, possibly carcinogenic to humans. The IARC conclusions are based on
evidence showing that high concentrations of pigment-grade and ultrafine TiO2
dust cause respiratory tract cancer in rats. Intrinsic cyto- and genotoxicity
of both TiO2 and ZnO NPs (<100 nm) has been frequently reported. Coating
the NPs does reduce the toxic effects, especially when silica-based coatings
are used, but cannot completely prevent these effects. TiO2,
however, has currently attracted more scientific attention than ZnO
[40,133,134].
Zinc oxide
Sunscreens containing metal oxide nanoparticles appear transparent on the
skin and provide excellent protection against sunburn caused by UV radiation.
Zinc oxide is a widely used broad-spectrum sunscreen, repeated application of
ZnO-NPs to the skin, as used in global sunscreen products, appears to be safe,
with no evidence of ZnO-NP penetration into the viable epidermis or toxicity in
the underlying viable epidermis. It was associated with the release and
penetration of zinc ions into the skin, but this did not appear to cause local
toxicity. In the past two decades, ZnO NPs have become one of the most popular
metal oxide nanoparticles in biological applications due to their excellent
biocompatibility, economic, and low toxicity. ZnO NPs have emerged a promising
potential in biomedicine, especially in the fields of anticancer and
antibacterial fields, which are involved with their potent ability to trigger
excess ROS production, release zinc ions, and induce cell apoptosis. In
addition, ZnO NPs have superior antibacterial, antimicrobial, and excellent
UV-blocking properties. ZnO NPs-exposed HepG2 cells presented higher
cytotoxicity and genotoxicity, which were associated with cell apoptosis
mediated by the ROS triggered mitochondrial pathway. In the brain, the
oxidative stress and inflammation reaction were found after ZnO NP exposure and
more remarkable changes were identified in aged individual. These kinds of
neurotoxicity might due to the destruction of the BBB integrity caused partly
by ZnO NPs-induced systemic inflammation [135-138].
SUNSCREEN FORMULATION
On the surface, sunscreen products are pretty simple. They consist of a
delivery vehicle containing one or more sunscreen active ingredients. When
applied to the skin, these sunscreen actives intercept solar UV rays before
they can damage the underlying skin. However, while conceptually simple, a
detailed analysis reveals that sunscreen formulations are quite complex,
requiring careful selection of sunscreen active and vehicle components to
control multiple performance and in-use parameters (Exhibit 9) [164].
Alpha-Tocopherol (Filtrosol)
has many protective actions, such as decreasing immunosuppression, erythema,
photoaging, and photocarcinogenesis [40]. Naturally occurring vitamin E is not
a single compound; instead, vitamin E is a group of molecules with related
structures, some of which may have unique properties in skin. After topical
application, vitamin E accumulates not only in cell membranes but also in the
extracellular lipid matrix of the stratum corneum, where vitamin E contributes
to antioxidant defenses. However, much of a topically applied dose of vitamin E
alone will be destroyed in the skin following exposure to UV light. This
suggests that although vitamin E is working as an antioxidant, it is unstable
on its own and easily lost from the skin. Thus, improving the stability of
topical applications with vitamin E is important [165]. Methyl Cellulose
(Methylester of cellulose) is used as an emulsifying, suspending and thickening
agent in cosmetics, pharmaceutics and the chemical industry. It’s a non-ionic
polymer, is highly hydrophilic and thus easily dissolves in cold and hot water
[166]. Ethyl alcohol is antimicrobial preservative; disinfectant; skin
penetrant; solvent. Cetyl and cetearyl are both derived from coconut and are
fatty alcohols. Typically, fatty alcohols are used as emollients and thickeners
in skin-care products. Fatty alcohols are not irritating and, in fact, can be
beneficial for dry skin. If LMW alcohols are high up on the ingredients list on
a bottle of sunscreen then they are going to dry out skin. However, Topical
application of 10% ethanol stimulates the proliferation of peritoneal tissue
explants – a semi in vivo wound model
– which can be interpreted as positive influence for stimulation of wound
healing by ethanol [167,168]. Oleyl alcohol is mainly used in topical
pharmaceutical formulations and is generally regarded as a nontoxic and non-irritant
material at the levels employed as an excipient. However, contact dermatitis
due to oleyl alcohol has been reported. It’s an antifoaming agent; dissolution
enhancer; emollient; emulsifying agent; skin penetrant; sustained-release
agent. SD alcohol 40 is a type of denatured alcohol used in cosmetics and
personal care products as an anti-foaming agent, astringent, antimicrobial
agent and a solvent. In addition to SD alcohol 40, the specially denatured
alcohols acceptable for use in cosmetics are SD Alcohol 23-A and SD Alcohol
40-B. As an astringent, SD alcohol 40 causes biological tissue to contract or
draw together. After topical application, astringents work on proteins called
keratins, which function to hold skin cells together to form a barrier. The
bonds between keratins are affected by temperature and pH, forming only when
skin is slightly acidic or cool. If the bonds break, the keratin molecules will
separate, causing the outer layer of skin to swell. Astringents cool the skin
and cause the bonds to reform. It is this process that produces the temporary
toning effect associated with astringents [169].
2-Ethoxyethyl-p-Methoxycinnamate (Giv Tan® F/Cinoxate) used as
sunscreen. The most common moisturizing ingredients are occlusive agents which
create a barrier that blocks water from escaping the skin. Ingredients like Petrolatum, Mineral Oil and
Dimethicone can all be used as occlusive agents. Humectants, which are
ingredients that attract water, are also added to lotions. Glycerin is the most
commonly used humectant. Finally, emollients are added to improve the feel of
the lotion on the skin. They can reduce the tackiness and greasiness caused by
the other moisturizing ingredients. Common emollients include coconut oil,
cetyl esters, and certain silicones. Sunscreen formulations are typically
thinner in viscosity than standard skin lotions (Exhibit 10) [170].
Morin is a yellow chemical
compound that can be isolated from Maclura
pomifera (Osage orange), Maclura
tinctoria (old fustic) and from leaves of Psidium guajava (common guava) [161]. Morin is a
pentahydroxyflavone that is 7-hydroxyflavonol bearing three additional hydroxy
substituents at positions 2' 4' and 5. It has a role as an antioxidant, a
metabolite, an antihypertensive agent, a hepatoprotective agent, a
neuroprotective agent, an anti-inflammatory agent, an antineoplastic agent, an
antibacterial agent, an EC 5.99.1.2 (DNA topoisomerase) inhibitor and an
angiogenesis modulating agent. It is a pentahydroxyflavone and a
7-hydroxyflavonol [162]. ZnO and TiO2 combinations are particularly
valuable because of their ability to filter both UVA and UVB radiations,
providing broader UVR protection than that observed with individual component
[95]. In 2018, the EWG reported that a large increase in these inorganic
filters with ~41% of sunscreens in the United States designated as mineral
only. This figure has more than doubled (from 17%) since 2007 [111]. Only two
sunscreen active ingredients approved in the US, avobenzone
(butylmethoxydibenzoylmethane) and zinc oxide (ZnO), provide true
broad-spectrum protection against UVA wavelengths >360 nm. Although
effective against shorter UVR wavelengths <360 nm, titanium dioxide TiO2
is also often believed to confer broad-spectrum protection and is substituted
for ZnO or avobenzone. Use of proper formulation strategies can ensure that
avobenzone losses are minimized to the extent that they have no impact on a
product's ability to deliver sustained protection, even over periods of
prolonged exposure to UVR (Figure 22) [163].
SUNSCREEN VS. CORAL
REEF
While covering less than 1% of the ocean surface, coral reefs provide
habitat for nearly one third of marine fish species as well as 10% of all fish
captured for human consumption. Coral reefs also provide major essential
benefits to people, like food production, tourism, biotechnology development,
and coastal protection. There is strong evidence that some sunscreen
ingredients, especially oxybenzone, are harmful to corals if the concentration
in water is high. In some situations, primarily related to the number of
swimmers and the geography of the shoreline, concentrations of oxybenzone far
exceed the levels shown to be harmful to corals [181]. In 2015, the non-profit
Haereticus Environmental Laboratory surveyed Trunk Bay beach on St. John, where
visitors ranged from 2,000 to 5,000 swimmers daily, and estimated over 6,000
pounds of sunscreen was deposited on the reef annually. The same year, it found
an average of 412 pounds of sunscreen was deposited daily on the reef at
Hanauma Bay, a popular snorkeling destination in Oahu (Hawaii) that draws an
average of 2,600 swimmers each day. Over the past three years, one-fifth of the
world’s coral reefs have died off — and there is a growing awareness that
sunscreen is playing a role. From 6,000 to 14,000 tons of sunscreen slide off
of humans into coral reef areas each year, exposing the gorgeous underwater
ecosystems to chemicals that can kill them. Global warming is the main reason
that coral reefs are dying — but sunscreens play a role, too [182]. Danovaro et
al. [184] revealed that sunscreens, by promoting viral infection, potentially
play an important role in coral bleaching in areas prone to high levels of
recreational use by humans. Coral bleaching has negative impacts on
biodiversity and functioning of reef ecosystems and their production of goods
and services. This increasing world-wide phenomenon is associated with
temperature anomalies, high irradiance, pollution, and bacterial diseases. Hard-coral
bleaching and the increase in viral abundance in seawater were also seen after
coral treatment with mitomycin C, an antibiotic commonly used to induce the
lytic cycle in latent viral infections [184]. Unfortunately, the World
Conservation Institute estimates that 20% of coral reefs are already destroyed,
another 25% are in great immediate threat, and another 25% will be threatened
by 2050 [185].
Epilogue
The skin stratum corneum acts as a barrier that isolates the organism
from the environment and avoids water loss from tissues. Such a sealed sheath
is essential for the physiological processes of the skin and of the entire
body, but poses problems for the topical delivery of substances to the tissues.
This kind of hindrance has been known since ancient times and has been
empirically approached, e.g. by the use of greasy or oily materials in skin
care applications. Technological achievements in the topical administrations of
medicaments and in skin care products have led to the development of vehicles and
penetration enhancer and to the design of technologically advanced
formulations. Although synthetic chemical structures have been abundantly used
in this field, traditional practices and scientific studies have shown through
the years that botanical sources can provide all kinds of elements for
vehiculating drugs and active compounds and for enhancing their permeation
through the skin. There is wide variability in price, SPF protection, and
product claims among commercially available sunscreens. Cosmetic elegance is
one of the most important positive features, followed by product performance.
Dermatologists should counsel patients that sunscreen products come with
numerous marketing claims and varying cosmetic applicability, all of which must
be balanced with adequate photoprotection. In addition, consumers should be
advised to select a product with broad-spectrum coverage, an SPF of 30 or
higher, and water and/or sweat resistance in the setting of water activities or
high ambient temperatures. UV filters are analyzed in their pure form by
ingredient suppliers and also by cosmetic companies that purchase those filters
for incorporation into their finished products. Once these UV filters are
included into cosmetic finished products, they also need to be analyzed for
both quality control and regulatory compliance. Use of sun screening agents is
beneficial in minimizing the occurrence of skin cancers in people with fair
skin. However, the same effect on Asian skin is debatable, as this skin type is
considered to be resistant to skin cancers. Sunscreen use is advisable in young
adults to prevent and minimize other photodamaging effects. Affordability and
proper application techniques are the challenges that must be addressed in
order to achieve regular sunscreen usage.
ARTICLE SUMMARY
Promoting sunscreen use is an integral part of prevention programs aimed
at reducing UV radiation-induced skin damage and skin cancers. Protection
against both UVB and UVA radiation is advocated. Despite the efforts of
physicians and regulatory authorities to spread awareness regarding sunburn,
skin cancer, and the benefits of regularly using sun screening agents,
treatment adherence is low. Providing cosmetically acceptable preparations and
educating people about following the application instructions, is a challenge
often faced by treating physicians. Pricing sunscreens reasonably and making
them water resistant and non-sticky are a few of the challenges faced by
manufacturers. Manufacturers must also consider sensitization reactions,
especially in those having eczema or photo dermatoses. Sun protection factor
(SPF) is affected by application density, water resistance and other factors.
An adequate SPF for an individual should be balanced to skin phenotype and
exposure habits. The correct use of sunscreens should be combined with the
avoidance of midday sun and the wearing of protective clothing and glasses, as
part of an overall sun protection regimen. The FDA approved sunscreen
ingredients are extensively reviewed. A clear and straightforward
recommendation on their safety and efficacy still remains a big challenge.
1.
Budzowski A.
Sunburn vs. sun poisoning, Web UPMC MyHealth Matters.
2.
Moan J,
Grigalavicius M, Dahlback A, Baturaite Z, Juzeniene A (2014)
Ultraviolet-radiation and health: optimal time for sun exposure. Adv Exp Med
Biol 810: 423-428.
3.
MedicineNet
(2018) Sunburn (sun poisoning) symptoms, pain relief and healing time,
medically.
4.
MedicineNet
(2012) Get rid of sunburn fast: Pain relief, blisters, peeling. Home Remedies.
5.
Koh D, Wang H,
Lee J, Chia KS, Lee HP, et al. (2003) Basal cell carcinoma, squamous cell
carcinoma and melanoma of the skin: Analysis of the Singapore Cancer Registry
data 1968-1997. Br J Dermatol 148: 1161-1166.
6.
Amaro-Ortiz A,
Yan B, D'Orazio JA (2014) Ultraviolet radiation, aging and the skin: Prevention
of damage by topical cAMP manipulation. Molecules 19: 6202-6919.
7.
Amblard P,
Leccia MT (1992) Skin diseases with photosensitivity. Rev Prat 42: 1365-1368.
8.
Zuba EB,
Koronowska S, Osmola-Mańkowska A, Jenerowicz D (2016) Drug-induced
photosensitivity. Acta Dermatovenerol Croat 24: 55-64.
9.
https://www.webmd.com/skin-problems-and-treatments/sun-poisoning#1
10.
Guerra KC,
Crane JS (2019) Skin cancer prevention. StatPearls [Internet]. Treasure Island
(FL): StatPearls Publishing. Available from http://www.ncbi.nlm.nih.gov/books/NBK519527/
11.
Guerra KC,
Crane JS (2018) Sunburn. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534837/
12.
McStay CM
(2018) What is the pathophysiology of sunburn? Medscape.
13.
(2019) Chapter
14 Diseases of the skin. In: Rick D. Kellerman, David Rakel. Conn's Current
Therapy. Elsevier Health Sciences, p: 1031.
14.
Azevedo M,
Bandeira L, Luza C, Lemos A, Bandeira F (2018) Vitamin D deficiency, skin
phototype, sun index and metabolic risk among patients with high rates of sun
exposure living in the tropics. J Clin Aesthet Dermatol 8: 15-18.
15.
Driscoll MS,
Wagner RF Jr. (2000) Clinical management of the acute sunburn reaction. Cutis 66:
53-58.
16.
Faurschou A,
Wulf HC (2008) Topical corticosteroids in the treatment of acute sunburn: A randomized,
double-blind clinical trial. Arch Dermatol 144: 620-624.
17.
Feily A,
Namazi MR (2009) Aloe vera in
dermatology: A brief review. G Ital Dermatol Venereol 144: 85-91.
18.
Puvabanditsin
P, Vongtongsri R (2005) Efficacy of Aloe vera
cream in prevention and treatment of sunburn and suntan. J Med Assoc Thai 88: S173-176.
19.
Tadicherla S,
Berman B (2006) Percutaneous dermal drug delivery for local pain control. Ther
Clin Risk Manag 2: 99-113.
20.
Moore DE
(2002) Drug-induced cutaneous photosensitivity: Incidence, mechanism, prevention
and management. Drug Saf 25: 345-372.
21.
Drucker AM,
Rosen CF (2011) Drug-induced photosensitivity: Culprit drugs, management and
prevention. Drug Saf 34: 821-837.
22.
Harth Y,
Rapoport M (1996) Photosensitivity associated with antipsychotics,
antidepressants and anxiolytics. Drug Saf 14: 252-259.
23.
Pile HD,
Nicolas D (2019) Isotretinoin. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525949/
24.
Hodgkiss-Harlow
CJ, Eichenfield LF, Dohil MA (2011) Effective monitoring of isotretinoin safety
in a pediatric dermatology population: A novel "patient symptom survey"
approach. J Am Acad Dermatol 65: 517-524.
25.
Das LM, Binko
AM, Traylor ZP, Peng H, Lu KQ (2019) Vitamin D improves sunburns by increasing
autophagy in M2 macrophages. Autophagy 15: 813-826.
26.
Scott JF, Das
LM, Ahsanuddin S, Qiu Y, Binko AM, et al. (2017) Oral vitamin D rapidly
attenuates inflammation from sunburn: An interventional study. J Invest
Dermatol 137: 2078-2086.
27.
Golmohammadzadeh
S, Imani F, Hosseinzadeh H, Jaafari MR (2011) Preparation, characterization and
evaluation of sun protective and moisturizing effects of nanoliposomes
containing safranal. Iran J Basic Med Sci 14: 521-533.
28.
Opene C, Chren
MM, Linos E (2017) Types of shade vary in protection just like sunscreens. JAMA
Dermatol 153: 1070-1071.
29.
Wolf R, Matz
H, Orion E, Lipozencić J (2003) Sunscreens - The ultimate cosmetic. Acta
Dermatovenerol Croat 11: 158-162.
30.
Latha MS,
Martis J, Shobha V, Sham Shinde R, Bangera S, et al. (2013) Sun screening
agents: A review. J Clin Aesthet Dermatol 6: 16-26.
31.
Rego D,
Fernandes L, Nascimento T, Grenha A (2010) Evaluation of a sunscreen during a
typical beach period. J Pharm Bioallied Sci 2: 47-50.
32.
Schalka S,
Steiner D, Ravelli FN, Steiner T, Terena AC (2014) Brazilian Society of
Dermatology. Brazilian consensus on photoprotection. An Bras Dermatol 89: 1-74.
33.
https://www.who.int/uv/uv_and_health/en/
34.
Volkovova K,
Bilanicova D, Bartonova A, Letašiová S, Dusinska M (2012) Associations between
environmental factors and incidence of cutaneous melanoma. Review. Environ
Health 11: S12.
35.
Engelsen O
(2010) The relationship between ultraviolet radiation exposure and vitamin D
status. Nutrients 2: 482-495.
36.
Diffey BL
(2002) Human exposure to solar ultraviolet radiation. J Cosmet Dermatol 1: 124-130.
37.
Ruszkiewicz
JA, Pinkas A, Ferrer B, Peres TV, Tsatsakis A, et al. (2017) Neurotoxic effect
of active ingredients in sunscreen products, a contemporary review. Toxicol Rep
4: 245-259.
38.
Kannan S, Lim
HW (2014) Photoprotection and vitamin D: A review. Photodermatol Photoimmunol
Photomed 30: 137-145.
39.
Pandika M
(2018) Looking to nature for new sunscreens. ACS Cent Sci 4: 788-790.
40.
Gabros S, Zito
PM (2019) Sunscreens and photoprotection. In: StatPearls [Internet]. Treasure
Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537164/
41.
(2019) FDA
aims to strengthen sunscreen rules. Medical Press (Health).
42.
Maddipatla M
(2019) US FDA proposes new regulations for over-the-counter sunscreens.
Reuters.
43.
Ripamonti E,
Allifranchini E, Todeschi S, Bocchietto E (2018) Endocrine disruption by
mixtures in topical consumer products. Cosmetics 5: 61.
44.
(2019) Sunscreen:
How to help protect your skin from the sun? Web US FDA.
45.
https://www.dailymail.co.uk/health/article-6731267/U-S-FDA-proposes-new-regulations-counter-sunscreens.html
46.
(2019)
National Center for Biotechnology Information. PubChem Database. 4-Aminobenzoic
acid, CID=978. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/978
47.
Kullavanijaya
P, Lim HW (2005) Photoprotection. J Am Acad Dermatol 52: 937-958.
48.
Tahiliani S,
Shirolikar M (2018) Viva voce on sunscreens. Indian J Drugs Dermatol 4: 92-96.
49.
Govindu PCV,
Hosamani B, Moi S, Venkatachalam D, Asha S, et al. (2019) Glutathione as a
photo-stabilizer of avobenzone: An evaluation under glass-filtered sunlight
using UV-spectroscopy. Photochem Photobiol Sci 18: 198-207.
50.
(2019)
National Center for Biotechnology Information. PubChem Database. Avobenzone,
CID=51040. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/51040
51.
Raffa RB,
Pergolizzi JV Jr, Taylor R Jr, Kitzen JM; NEMA Research Group (2018) Sunscreen
bans: Coral reefs and skin cancer. J Clin Pharm Ther 44: 134-139.
52.
Aparici-Espert
I, Miranda MA, Lhiaubet-Vallet V (2018) Sunscreen-based photocages for topical
drugs: A photophysical and photochemical study of a diclofenac-avobenzone dyad.
Molecules 23: pii: E673.
53.
Yang C, Lim W,
Bazer FW, Song G (2018) Avobenzone suppresses proliferative activity of human
trophoblast cells and induces apoptosis mediated by mitochondrial disruption.
Reprod Toxicol 81: 50-57.
54.
Uco DP,
Leite-Silva VR, Silva HDT, Duque MD, Grice J (2018) UVA and UVB formulation
phototoxicity in a three-dimensional human skin model: Photodegradation effect.
Toxicol In Vitro 53: 37-44.
55.
Monteiro MS,
Ozzetti RA, Vergnanini AL, de Brito-Gitirana L, Volpato NM, et al. (2012)
Evaluation of octyl p-methoxycinnamate included in liposomes and cyclodextrins
in anti-solar preparations: Preparations, characterizations and in vitro penetration studies. Int J
Nanomed 7: 3045-3058.
56.
Lin YC, Lin
CF, Alalaiwe A, Wang PW, Fang YP, et al. (2018) UV filters entrapment in
mesoporous silica hydrogel for skin protection against UVA with minimization of
percutaneous absorption. Eur J Pharm Sci 122: 185-194.
57.
De Coster S,
van Larebeke N (2012) Endocrine-disrupting chemicals: Associated disorders and
mechanisms of action. J Environ Public Health 2012: 713696.
58.
Nash JF,
Tanner PR (2005) Chapter 9. Sunscreens. In: Draelos ZD, Thaman LA. Cosmetic
Formulation of Skin Care Products Cosmetic Science and Technology. Taylor &
Francis, p: 139.
59.
(2004) Chapter
4. Sun Sense, Avobenzone under Fire. In: Paula Begoun. The Complete Beauty
Bible: The Ultimate Guide to Smart Beauty. Rodale, ISBN1579549993,
9781579549992.
60.
Ainbinder D,
Touitou E (2009) Chapter 44. Skin Photodamage Prevention: State-of-the-art and
new prospects. In: Farage MA, Miller KW, Maibach HI. Textbook of Aging Skin.
Springer Science & Business Media, p: 436.
61.
Gunia-Krzyżak
A, Słoczyńska K, Popiół J, Koczurkiewicz P, Marona H, et al. (2018) Cinnamic
acid derivatives in cosmetics: Current use and future prospects. Int J Cosmet
Sci 40: 356-366.
62.
Rai R,
Shanmuga SC, Srinivas C (2012) Update on photoprotection. Indian J Dermatol 57:
335-342.
63.
Rao GS, Tokuda
H, Ichiishi E, Takasaki M, Iida A, et al. (2013) Oral chemoprevention of skin
cancer in mice by benzophenone sunscreens dioxybenzone and octabenzone in
drinking water. Anticancer Res 33: 2535-2540.
64.
Heo S, Hwang
HS, Jeong Y, Na K (2018) Skin protection efficacy from UV irradiation and skin
penetration property of polysaccharide-benzophenone conjugates as a sunscreen
agent. Carbohydr Polym 195: 534-541.
65.
Sherwood VF,
Kennedy S, Zhang H, Purser GH, Sheaff RJ (2012) Altered UV absorbance and
cytotoxicity of chlorinated sunscreen agents. Cutan Ocul Toxicol 31: 273-279.
66.
Butler TC
(1948) Bromal hydrate and chloral hydrate: A pharmacological contrast and its
chemical basis. J Pharmacol Exp Ther 94: 401-411.
67.
Fediuk DJ
(1993) Pharmacokinetic and toxicological characterization of repellent DEET and
sunscreen oxybenzone (Thesis Submitted for PhD). Available from: https://mspace.lib.umanitoba.ca/bitstream/handle/1993/8112/fediuk_daryl.pdf?sequence=1
68.
Seto Y, Ohtake
H, Kato M, Onoue S (2015) Phototoxic risk assessments on benzophenone
derivatives: photobiochemical assessments and dermal cassette-dosing
pharmacokinetic study. J Pharmacol Exp Ther 354: 195-202.
69.
Ngan V (2012)
Allergy to benzophenone. Web DermNet NZ.
70.
(2019)
National Center for Biotechnology Information. PubChem Database. Homosalate,
CID=8362. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/8362
71.
Kim TH, Shin
BS, Kim KB, Shin SW, Seok SH, et al. (2014) Percutaneous absorption, disposition
and exposure assessment of homosalate, a UV filtering agent, in rats. J Toxicol
Environ Health A 77: 202-213.
72.
Kullavanijaya
P, Lim HW (2005) Photoprotection. J Am Acad Dermatol 52: 937-958.
73.
Erol M, Çok I,
Bostan Gayret Ö, Günes P, Yigit Ö, et al. (2017) Evaluation of the
endocrine-disrupting effects of homosalate (HMS) and 2-ethylhexyl
4-dimethylaminobenzoate (OD-PABA) in rat pups during the prenatal, lactation
and early postnatal periods. Toxicol Ind Health 33: 775-791.
74.
Wang J, Pan L,
Wu S, Lu L, Xu Y, et al. (2016) Recent advances on endocrine disrupting effects
of UV filters. Int J Environ Res Public Health 13: pii: E782.
75.
http://www.safecosmetics.org/get-the-facts/chemicals-of-concern/homosalate/
76.
Rodrigues NDN,
Cole-Filipiak NC, Blodgett KN, Abeysekera C, Zwier TS, et al. (2018) Wave packet
insights into the photoprotection mechanism of the UV filter methyl
anthranilate. Nat Commun 9: 5188.
77.
Dale Wilson B,
Moon S, Armstrong F (2012) Comprehensive review of ultraviolet radiation and
the current status on sunscreens. J Clin Aesthet Dermatol 5: 18-23.
78.
Sivamani RK,
Crane LA, Dellavalle RP (2009) The benefits and risks of ultraviolet tanning
and its alternatives: the role of prudent sun exposure. Dermatol Clin 27: 149-154.
79.
Pinto I, Bogi
A, Picciolo F, Stacchini N, Buonocore G, et al. (2015) Blue light and
ultraviolet radiation exposure from infant phototherapy equipment. J Occup
Environ Hyg 12: 603-610.
80.
De Groot T,
Chowdhary A, Meis JF, Voss A (2019) Killing of Candida auris by UV-C: Importance of exposure time and distance.
Mycoses 62: 408-412.
81.
Dai T,
Kharkwal GB, Zhao J, St Denis TG, Wu Q, et al. (2011) Ultraviolet-C light for
treatment of Candida albicans burn
infection in mice. Photochem Photobiol 87: 342-349.
82.
Nussbaumer B,
Kaminski-Hartenthaler A, Forneris CA, Morgan LC, Sonis JH, et al. (2015) Light
therapy for preventing seasonal affective disorder. Cochrane Database Syst Rev 11:
CD011269.
83.
Lam RW, Levitt
AJ, Levitan RD, Michalak EE, Cheung AH, et al. (2016) Efficacy of bright light
treatment, fluoxetine and the combination in patients with nonseasonal major
depressive disorder: A randomized clinical trial. JAMA Psychiatry 73: 56-63.
84.
Guy GP Jr,
Thomas CC, Thompson T, Watson M, Massetti GM, et al. (2015) Vital signs: Melanoma
incidence and mortality trends and projections - United States, 1982-2030. MMWR
Morb Mortal Wkly Rep 64: 591-596.
85.
Sample A, He
YY (2018) Mechanisms and prevention of UV-induced melanoma. Photodermatol
Photoimmunol Photomed 34: 13-24.
86.
Mackiewicz-Wysocka
M, Bowszyc-Dmochowska M, Strzelecka-Węklar D, Dańczak-Pazdrowska A, Adamski Z (2013)
Basal cell carcinoma - Diagnosis. Contemp Oncol (Pozn) 17: 337-342.
87.
(2007) Sunscreen
options. In: Wolverton SE, Comprehensive Dermatologic Drug Therapy, Edn 2.
Elsevier Health Sciences, ISBN 1437720706, 9781437720709.
88.
(2016) Chapter
9. Photoallergens. In: Marks Jr JG, Anderson BE and DeLeo VA, Contact &
Occupational Dermatology. JP Medical Ltd., ISBN 9351529363, 9789351529361.
89.
Moyal D,
Chardon A, Kollias N (2000) UVA protection efficacy of sunscreens can be
determined by the persistent pigment darkening (PPD) method. (Part 2).
Photodermatol Photoimmunol Photomed 16: 250-255.
90.
Wang SQ, Xu H,
Stanfield JW, Osterwalder U, Herzog B (2017) Comparison of ultraviolet A light
protection standards in the United States and European Union through in vitro measurements of commercially
available sunscreens. J Am Acad Dermatol 77: 42-47.
91.
Schalka S,
Naranjo Ravelli F, Perim N, Vasconcelos R (2017) Chemical and physical
sunscreens. In: Issa M, Tamura B (eds). Daily Routine in Cosmetic Dermatology.
Clin Approaches Procedures Cosmetic Dermatol 1. Springer, Cham. ISBN
978-3-319-12588-6, 978-3-319-12589-3.
92.
Jallad KN
(2017) Chemical characterization of sunscreens composition and its related
potential adverse health effects. J Cosmet Dermatol 16: 353-357.
93.
(2016)
Protecting your skin. Chemical vs. physical (mineral) sunscreens: Pros and cons.
Blog Dr. Desjarlais.
94.
Moloney FJ,
Collins S, Murphy GM (2002) Sunscreens: Safety, efficacy and appropriate use.
Am J Clin Dermatol 3: 185-191.
95.
Shetty PK,
Venuvanka V, Jagani HV, Chethan GH, Ligade VS, et al. (2015) Development and
evaluation of sunscreen creams containing morin-encapsulated nanoparticles for
enhanced UV radiation protection and antioxidant activity. Int J Nanomed 10: 6477-6491.
96.
Kumar R, Deep
G, Agarwal R (2015) An overview of ultraviolet B radiation-induced skin cancer
chemoprevention by silibinin. Curr Pharmacol Rep 1: 206-215.
97.
Levy SB (2015)
Chapter 29. UV Filters. In: Barel AO, Paye M, Maibach HI. Handbook of Cosmetic
Science and Technology. CRC Press, ISBN 1420069683, 9781420069686.
98.
Avenel-Audran
M, Dutartre H, Goossens A, Jeanmougin M, Comte C, et al. (2010) Octocrylene, an
emerging photoallergen. Arch Dermatol 146: 753-757.
99.
De Groot AC,
Roberts DW (2014) Contact and photocontact allergy to octocrylene: A review.
Contact Dermatitis 70: 193-204.
100.
Berkman MS,
Yazan Y (2012) Solid lipid nanoparticles: A possible vehicle for zinc oxide and
octocrylene. Pharmazie 67: 202-208.
101.
Karlsson I,
Vanden Broecke K, Mårtensson J, Goossens A, Börje A (2011) Clinical and
experimental studies of octocrylene's allergenic potency. Contact Dermatitis
64: 343-352.
102.
Briasco B,
Capra P, Mannucci B, Perugini P (2017) Stability study of sunscreens with free
and encapsulated UV filters contained in plastic packaging. Pharmaceutics 9:
pii: E19.
103.
Manová E, von
Goetz N, Hungerbühler K (2014) Ultraviolet filter contact and photocontact
allergy: Consumer exposure and risk assessment for octocrylene from personal
care products and sunscreens. Br J Dermatol 171: 1368-1374.
104.
Blüthgen N,
Meili N, Chew G, Odermatt A, Fent K (2014) Accumulation and effects of the
UV-filter octocrylene in adult and embryonic zebrafish (Danio rerio). Sci Total
Environ 476-477: 207-217.
105.
(2019)
National Center for Biotechnology Information. PubChem Database. Octinoxate,
CID=5355130. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/5355130
106.
Lim HW, Thomas
L, Rigel DS (2004) Chapter 6. Photoprotection. In: Rigel DS, Weiss RA, Lim HW
and Dover JS. Photoaging Basic and Clinical Dermatology. CRC Press, ISBN 0824752090, 9780824752095.
107.
(2018) Molecule
of the Week Archive. In a few years, coral in Hawaiian waters will no longer
have to put up with us. What molecules are we? Web ACS.
108.
Siller A,
Blaszak SC, Lazar M, Olasz Harken E (2018) Update about the effects of the
sunscreen ingredients oxybenzone and octinoxate on humans and the environment.
Plast Surg Nurs 38: 158-161.
109.
Raffa RB,
Pergolizzi JV Jr, Taylor R Jr, Kitzen JM; NEMA Research Group (2019) Sunscreen
bans: Coral reefs and skin cancer. J Clin Pharm Ther 44: 134-139.
110.
(2019)
Cinoxate market is likely to witness huge growth – Major key players are
Parchem (US), Carbosynth Limited (UK), Synchem UG & Co. KG (DE). Market
Research Gazette.
111.
Schneider SL,
Lim HW (2018) A review of inorganic UV filters zinc oxide and titanium dioxide.
Photodermatol Photoimmunol Photomed.
112.
Axelstad M,
Boberg J, Hougaard KS, Christiansen S, Jacobsen PR, et al. (2011) Effects of
pre- and post-natal exposure to the UV-filter octyl methoxycinnamate (OMC) on
the reproductive, auditory and neurological development of rat offspring.
Toxicol Appl Pharmacol 250: 278-290.
113.
Darbre PD
(2006) Environmental oestrogens, cosmetics and breast cancer. Best Pract Res
Clin Endocrinol Metab 20: 121-143.
114.
Wang Y,
Marling SJ, Plum LA, DeLuca HF (2017) Salate derivatives found in sunscreens
block experimental autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S
A 114: 8528-8531.
115.
Michalun MV,
DiNardo JC (2014) Skin care and cosmetic ingredients dictionary. Cengage
Learning, ISBN 1305178211, 9781305178212.
116.
(2019)
National Center for Biotechnology Information. PubChem Database. Oxybenzone,
CID=4632. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/4632
117.
DiNardo JC,
Downs CA (2018) Dermatological and environmental toxicological impact of the
sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol 17: 15-19.
118.
Sung CR, Kim
KB, Lee JY, Lee BM, Kwack SJ (2019) Risk assessment of ethylhexyl dimethyl PABA
in cosmetics. Toxicol Res 35: 131-136.
119.
Draelos ZZ (2008)
Procedures in cosmetic dermatology. Edition 2. Elsevier Health Sciences, ISBN
1437720943, 9781437720945.
120.
Bastien N,
Millau JF, Rouabhia M, Davies RJ, Drouin R (2010) The sunscreen agent
2-phenylbenzimidazole-5-sulfonic acid photosensitizes the formation of oxidized
guanines in cellulose after UV-A or UV-B exposure. J Invest Dermatol 130: 2463-2471.
121.
Inbaraj JJ,
Bilski P, Chignell CF (2002) Photophysical and photochemical studies of
2-phenylbenzimidazole and UVB sunscreen 2-phenylbenzimidazole-5-sulfonic acid.
Photochem Photobiol 75: 107-116.
122.
Rai R,
Shanmuga SC, Srinivas C (2012) Update on photoprotection. Indian J Dermatol 57:
335-342.
123.
Rigel DS,
Friedman R, Robinson JK, Ross MI, Cockerell CJ, et al. (2011) Cancer of the skin
e-book: Expert consult. Edition 2. Elsevier Health Sciences, ISBN 1437736149,
9781437736144.
124.
(2006)
Approved Drug Products and Legal Requirements. Volume 3 of USP DI: V. 3 of USP,
United States Pharmacopeial Convention Inc. Thomson Micromedex, ISBN
1563635763, 9781563635762.
125.
(2005) USP DI:
Approved Drug Products and Legal Requirement USP DI VOL 3. Thomson/MICROMEDEX,
ISBN 156363516X, 9781563635168.
126.
Lin EW,
Boehnke N, Maynard HD (2014) Protein-polymer conjugation via ligand affinity
and photoactivation of glutathione S-transferase. Bioconjug Chem 25: 1902-1909.
127.
Hermanson GT
(2013) Chapter 18 - PEGylation and synthetic polymer modification. In:
Hermanson G. Bioconjugate Techniques (3rd Edn), pp: 787-838.
128.
US FDA
Database. CFR - Code of Federal Regulations Title 21
129.
Lowe NJ (1996)
Sunscreens: Development: Evaluation, and regulatory aspects. 2nd Edn,
Cosmetic Science and Technology Series. CRC Press, ISBN 0824793064,
9780824793067.
130.
Kaur A, Thatai
P, Sapra B (2014) Need of UV protection and evaluation of efficacy of
sunscreens. J Cosmet Sci 65: 315-345.
131.
(2019) National
Center for Biotechnology Information. PubChem Database. Trolamine salicylate,
CID=25213. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/25213
132.
http://dermapproved.com/active-ingredients/trolamine-salicylate
133.
Smijs TG,
Pavel S (2011) Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus
on their safety and effectiveness. Nanotechnol Sci Appl 4: 95-112.
134.
Trivedi M,
Murase J (2017) Chapter 4. Titanium Dioxide in Sunscreen. In: Janus M.
Application of Titanium Dioxide. Intech Open, ISBN 9535134299, 9789535134299.
135.
Mohammed YH,
Holmes A, Haridass IN, Sanchez WY, Studier H, et al. (2019) Support for the safe
use of zinc oxide nanoparticle sunscreens: Lack of skin penetration or cellular
toxicity after repeated application in volunteers. J Invest Dermatol 139: 308-315.
136.
Wright PFA
(2019) Realistic exposure study assists risk assessments of ZnO nanoparticle
sunscreens and allays safety concerns. J Invest Dermatol 139: 277-278.
137.
Jiang J, Pi J,
Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical
applications. Bioinorg Chem Appl 1062562.
138.
Tian L, Lin B,
Wu L, Li K, Liu H, et al. (2015) Neurotoxicity induced by zinc oxide
nanoparticles: Age-related differences and interaction. Sci Rep 5: 16117.
139.
Schwarz T
(2005) Mechanisms of UV-induced immunosuppression. Keio J Med 54: 165-171.
140.
Ullrich SE
(2005) Mechanisms underlying UV-induced immune suppression. Mutat Res 571: 185-205.
141.
Katiyar SK
(2007) UV-induced immune suppression and photocarcinogenesis: chemoprevention
by dietary botanical agents. Cancer Lett 255: 1-11.
142.
Cho BA, Yoo
SK, Seo JS (2018) Signatures of photo-aging and intrinsic aging in skin were
revealed by transcriptome network analysis. Aging (Albany NY) 10: 1609-1626.
143.
Bosch R,
Philips N, Suárez-Pérez JA, Juarranz A, Devmurari A, et al. (2015) Mechanisms
of photoaging and cutaneous photocarcinogenesis and photoprotective strategies
with phytochemicals. Antioxidants (Basel) 4: 248-268.
144.
Kang S, Fisher
GJ, Voorhees JJ (2001) Photoaging: Pathogenesis, prevention and treatment. Clin
Geriatr Med 17: 643-659.
145.
Han A, Chien
AL, Kang S (2014) Photoaging. Dermatol Clin 32: 291-299, vii.
146.
Sjerobabski
Masnec I, Poduje S (2008) Photoaging. Coll Antropol 32: 177-180.
147.
Fisher GJ,
Kang S, Varani J (2002) Mechanisms of photoaging and chronological skin aging.
Arch Dermatol 138: 1462-1470.
148.
Rabe JH,
Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN (2006) Photoaging: Mechanisms and
repair. J Am Acad Dermatol 55:1-19.
149.
Rittié L,
Fisher GJ (2015) Natural and sun-induced aging of human skin. Cold Spring Harb
Perspect Med 5: a015370.
150.
Amano S (2016)
Characterization and mechanisms of photoageing-related changes in skin. Damages
of basement membrane and dermal structures. Exp Dermatol 3: 14-19.
151.
Pamela RD
(2018) Topical growth factors for the treatment of facial photoaging: A clinical
experience of eight cases. J Clin Aesthet Dermatol 11: 28-29.
152.
Dhaliwal S,
Rybak I, Ellis SR, Notay M, Trivedi M, et al. (2019) Prospective, randomized,
double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol 180: 289-296.
153.
Mukherjee S,
Date A, Patravale V, Korting HC, Roeder A, et al. (2006) Retinoids in the
treatment of skin aging: An overview of clinical efficacy and safety. Clin
Interv Aging 1: 327-348.
154.
Pandel R,
Poljšak B, Godic A, Dahmane R (2013) Skin photoaging and the role of
antioxidants in its prevention. ISRN Dermatol 2013: 930164.
155.
Sivamani RK,
Crane LA, Dellavalle RP (2009) The benefits and risks of ultraviolet tanning
and its alternatives: the role of prudent sun exposure. Dermatol Clin 27: 149-154,
vi.
156.
Juzeniene A,
Moan J (2012) Beneficial effects of UV radiation other than via vitamin D
production. Dermatoendocrinol 4: 109-117.
157.
Choi W,
Miyamura Y, Wolber R, Smuda C, Reinhold W, et al. (2010) Regulation of human
skin pigmentation in situ by repetitive UV exposure: Molecular characterization
of responses to UVA and/or UVB. J Invest Dermatol 130: 1685-1696.
158.
Brenner M,
Hearing VJ (2008) The protective role of melanin against UV damage in human
skin. Photochem Photobiol 84: 539-549.
159.
Hwang YJ, Park
HJ, Hahn HJ, Kim JY, Ko JH, et al. (2011) Immediate pigment darkening and
persistent pigment darkening as means of measuring the ultraviolet A protection
factor in vivo: A comparative study.
Br J Dermatol 164: 1356-1361.
160.
Del Bino S,
Duval C, Bernerd F (2018) Clinical and biological characterization of skin
pigmentation diversity and its consequences on UV impact. Int J Mol Sci 19:
pii: E2668.
161.
Rattanachaikunsopon
P, Phumkhachorn P (2007) Bacteriostatic effect of flavonoids isolated from
leaves of Psidium guajava on fish
pathogens. Fitoterapia 78: 434-436.
162.
(2019)
National Center for Biotechnology Information. PubChem Database. Morin,
CID=5281670. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/5281670
163.
Beasley DG,
Meyer TA (2010) Characterization of the UVA protection provided by avobenzone,
zinc oxide and titanium dioxide in broad-spectrum sunscreen products. Am J Clin
Dermatol 11: 413-421.
164.
Tanner PR (2006)
Sunscreen product formulation. Dermatol Clin 24: 53-62.
165.
Weber C, Podda
M, Rallis M, Thiele JJ, Traber MG, et al. (1997) Efficacy of topically applied
tocopherols and tocotrienols in protection of murine skin from oxidative damage
induced by UV-irradiation. Free Radic Biol Med 22: 761-769.
166.
(2012) Chapter
2. Raw materials of cosmetics. In: Iwata H, Shimada K. Formulas, Ingredients
and Production of Cosmetics: Technology of Skin- and Hair-Care Products in
Japan. Springer Science & Business Media, ISBN 4431540601, 9784431540601.
167.
(2009) Alcohol
(Page 17). In: Rowe RC, Sheskey PJ, Quinn ME. Handbook of Pharmaceutical
Excipients, Edition 6. Pharmaceutical Press, ISBN 1582121354, 9781582121352.
168.
Lachenmeier DW
(2008) Safety evaluation of topical applications of ethanol on the skin and
inside the oral cavity. J Occup Med Toxicol 3: 26.
169.
https://thedermreview.com/sd-alcohol-40/
170.
https://chemistscorner.com/cosmetic-formulation-basics-sunscreens/
171.
Habbema L,
Halk AB, Neumann M, Bergman W (2017) Risks of unregulated use of
alpha-melanocyte-stimulating hormone analogues: A review. Int J Dermatol 56: 975-980.
172.
Garone M,
Howard J, Fabrikant J (2015) A review of common tanning methods. J Clin Aesthet
Dermatol 8: 43-47.
173.
Ciriminna R,
Fidalgo A, Ilharco LM, Pagliaro M (2018) Dihydroxyacetone: An updated insight
into an important bioproduct. ChemistryOpen 7: 233-236.
174.
Martini MC
(2017) Self-tanning and sunless tanning products. Ann Dermatol Venereol 144: 638-644.
175.
Holman DM, Fox
KA, Glenn JD, Guy GP Jr, Watson M, et al. (2013) Strategies to reduce indoor
tanning: Current research gaps and future opportunities for prevention. Am J
Prev Med 44: 672-681.
176.
(2008) Give me
a tan! - Megan Fox. News24.com Archives.
177.
March B (2014)
10 celebs who look better tan-free. COSMOPOLITAN.
178.
Haggan M
(2018) Warning on incorrect sunscreen use. Austr J Pharm.
179.
Grand View
Research (2018-2025) U.S. Sun Care Market Size, Share, Industry Trends Report.
180.
(2018)
Sunscreen Manufacturing Industry in the US. IBISWorld.
181.
Zirwas MJ,
Andrasik W (2018) Can sunscreens harm coral reefs? Addressing environmental
concerns and offering practical recommendations. Skinmed 16: 223-229.
182.
Belluz J
(2018) Hawaii is banning sunscreens that kill coral reefs. Vox.
183.
Reef-safe
Sunscreen: What You Need to Know. Blog CHASING CORAL.
184.
Danovaro R,
Bongiorni L, Corinaldesi C, Giovannelli D, Damiani E, et al. (2008) Sunscreens
cause coral bleaching by promoting viral infections. Environ Health Perspect 116:
441-447.
185.
Leonardo
DiCaprio Foundation. Replenishing Coral Reefs in the Pacific Ocean.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of AIDS (ISSN: 2644-3023)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Renal Transplantation Science (ISSN:2640-0847)